Input interpretation

oxidants | Hill formula
Table
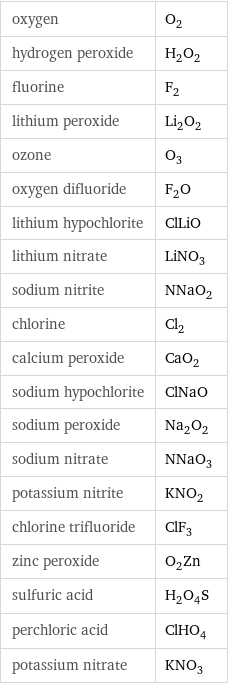
oxygen | O_2 hydrogen peroxide | H_2O_2 fluorine | F_2 lithium peroxide | Li_2O_2 ozone | O_3 oxygen difluoride | F_2O lithium hypochlorite | ClLiO lithium nitrate | LiNO_3 sodium nitrite | NNaO_2 chlorine | Cl_2 calcium peroxide | CaO_2 sodium hypochlorite | ClNaO sodium peroxide | Na_2O_2 sodium nitrate | NNaO_3 potassium nitrite | KNO_2 chlorine trifluoride | ClF_3 zinc peroxide | O_2Zn sulfuric acid | H_2O_4S perchloric acid | ClHO_4 potassium nitrate | KNO_3