Input interpretation
zinc chlorate | vanadium(II) oxide
Chemical names and formulas
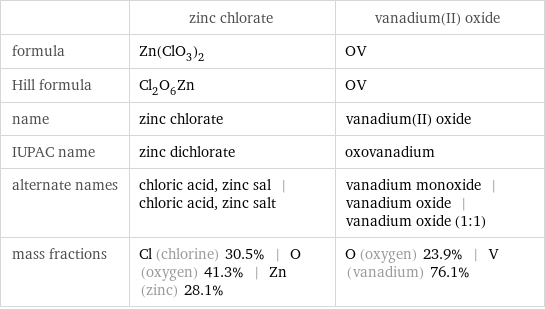
| zinc chlorate | vanadium(II) oxide formula | Zn(ClO_3)_2 | OV Hill formula | Cl_2O_6Zn | OV name | zinc chlorate | vanadium(II) oxide IUPAC name | zinc dichlorate | oxovanadium alternate names | chloric acid, zinc sal | chloric acid, zinc salt | vanadium monoxide | vanadium oxide | vanadium oxide (1:1) mass fractions | Cl (chlorine) 30.5% | O (oxygen) 41.3% | Zn (zinc) 28.1% | O (oxygen) 23.9% | V (vanadium) 76.1%
Structure diagrams
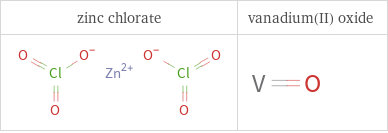
Structure diagrams
Basic properties
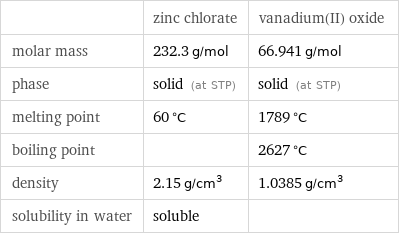
| zinc chlorate | vanadium(II) oxide molar mass | 232.3 g/mol | 66.941 g/mol phase | solid (at STP) | solid (at STP) melting point | 60 °C | 1789 °C boiling point | | 2627 °C density | 2.15 g/cm^3 | 1.0385 g/cm^3 solubility in water | soluble |
Units

Thermodynamic properties
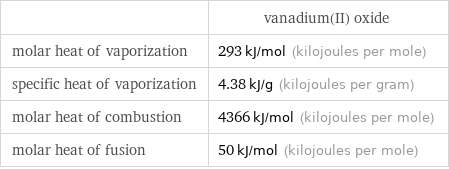
| vanadium(II) oxide molar heat of vaporization | 293 kJ/mol (kilojoules per mole) specific heat of vaporization | 4.38 kJ/g (kilojoules per gram) molar heat of combustion | 4366 kJ/mol (kilojoules per mole) molar heat of fusion | 50 kJ/mol (kilojoules per mole)
Solid properties (at STP)

| zinc chlorate | vanadium(II) oxide density | 2.15 g/cm^3 | 1.0385 g/cm^3 refractive index | | 1.5763
Units

Chemical identifiers
![| zinc chlorate | vanadium(II) oxide CAS number | 10361-95-2 | 12035-98-2 PubChem CID number | 25206 | 24411 SMILES identifier | [O-]Cl(=O)=O.[O-]Cl(=O)=O.[Zn+2] | O=[V] InChI identifier | InChI=1/2ClHO3.Zn/c2*2-1(3)4;/h2*(H, 2, 3, 4);/q;;+2/p-2/f2ClO3.Zn/q2*-1;m | InChI=1/O.V/rOV/c1-2 EU number | 233-804-7 | 234-834-3 Gmelin number | 39123 | 13746](../image_source/9ee14703069deaadcb7458c470dbed67.png)
| zinc chlorate | vanadium(II) oxide CAS number | 10361-95-2 | 12035-98-2 PubChem CID number | 25206 | 24411 SMILES identifier | [O-]Cl(=O)=O.[O-]Cl(=O)=O.[Zn+2] | O=[V] InChI identifier | InChI=1/2ClHO3.Zn/c2*2-1(3)4;/h2*(H, 2, 3, 4);/q;;+2/p-2/f2ClO3.Zn/q2*-1;m | InChI=1/O.V/rOV/c1-2 EU number | 233-804-7 | 234-834-3 Gmelin number | 39123 | 13746
NFPA label

NFPA label
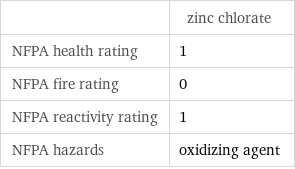
| zinc chlorate NFPA health rating | 1 NFPA fire rating | 0 NFPA reactivity rating | 1 NFPA hazards | oxidizing agent