Input interpretation

f-block elements | atomic diameter
Summary

median | 360 pm highest | 390 pm (lanthanum and actinium) lowest | 350 pm (10 elements) distribution | | (based on 21 values; 7 unavailable)
Entities with missing values

curium | berkelium | californium | einsteinium | fermium | mendelevium | nobelium (total: 7)
Plot
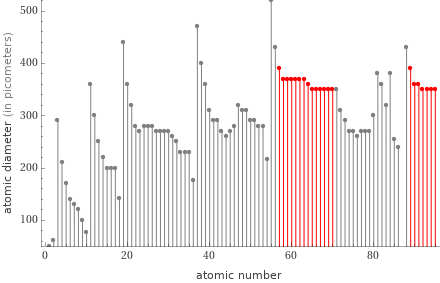
Plot
Periodic table location
Periodic table location
Atomic radii properties
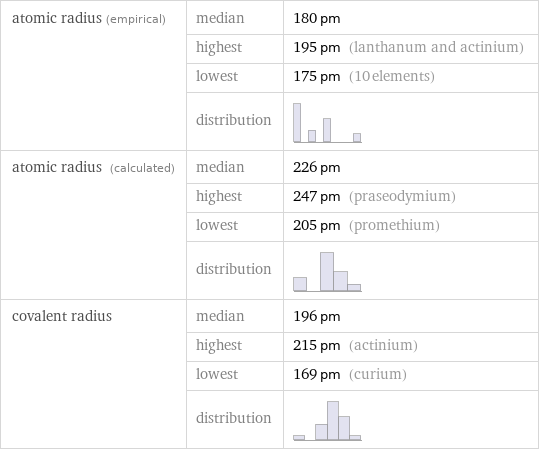
atomic radius (empirical) | median | 180 pm | highest | 195 pm (lanthanum and actinium) | lowest | 175 pm (10 elements) | distribution | atomic radius (calculated) | median | 226 pm | highest | 247 pm (praseodymium) | lowest | 205 pm (promethium) | distribution | covalent radius | median | 196 pm | highest | 215 pm (actinium) | lowest | 169 pm (curium) | distribution |
Units

Atomic diameter rankings
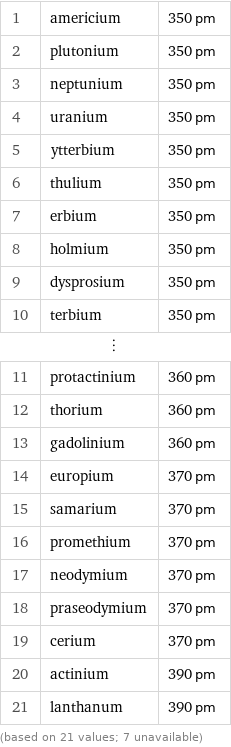
1 | americium | 350 pm 2 | plutonium | 350 pm 3 | neptunium | 350 pm 4 | uranium | 350 pm 5 | ytterbium | 350 pm 6 | thulium | 350 pm 7 | erbium | 350 pm 8 | holmium | 350 pm 9 | dysprosium | 350 pm 10 | terbium | 350 pm ⋮ | | 11 | protactinium | 360 pm 12 | thorium | 360 pm 13 | gadolinium | 360 pm 14 | europium | 370 pm 15 | samarium | 370 pm 16 | promethium | 370 pm 17 | neodymium | 370 pm 18 | praseodymium | 370 pm 19 | cerium | 370 pm 20 | actinium | 390 pm 21 | lanthanum | 390 pm (based on 21 values; 7 unavailable)
Unit conversions for median atomic diameter 360 pm
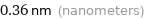
0.36 nm (nanometers)
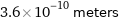
3.6×10^-10 meters
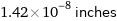
1.42×10^-8 inches
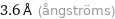
3.6 Å (ångströms)
Comparisons for median atomic diameter 360 pm

≈ 0.4 × carbon nanotube diameter ( ≈ 1 nm )
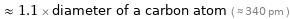
≈ 1.1 × diameter of a carbon atom ( ≈ 340 pm )
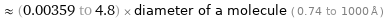
≈ (0.00359 to 4.8) × diameter of a molecule ( 0.74 to 1000 Å )