Input interpretation
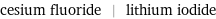
cesium fluoride | lithium iodide
Chemical names and formulas
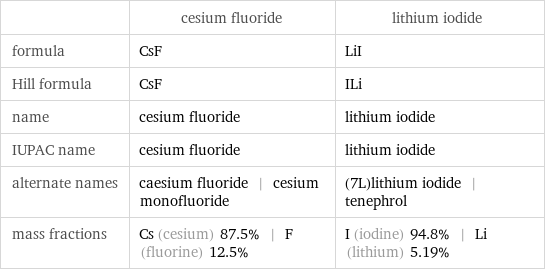
| cesium fluoride | lithium iodide formula | CsF | LiI Hill formula | CsF | ILi name | cesium fluoride | lithium iodide IUPAC name | cesium fluoride | lithium iodide alternate names | caesium fluoride | cesium monofluoride | (7L)lithium iodide | tenephrol mass fractions | Cs (cesium) 87.5% | F (fluorine) 12.5% | I (iodine) 94.8% | Li (lithium) 5.19%
Structure diagrams

Structure diagrams
Basic properties
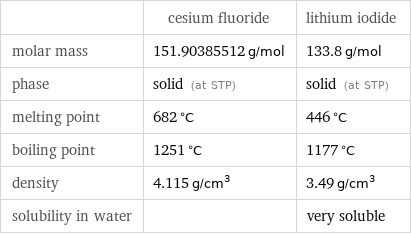
| cesium fluoride | lithium iodide molar mass | 151.90385512 g/mol | 133.8 g/mol phase | solid (at STP) | solid (at STP) melting point | 682 °C | 446 °C boiling point | 1251 °C | 1177 °C density | 4.115 g/cm^3 | 3.49 g/cm^3 solubility in water | | very soluble
Units

Thermodynamic properties
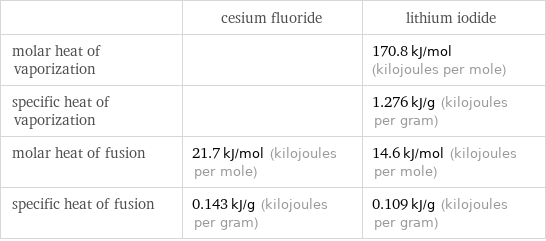
| cesium fluoride | lithium iodide molar heat of vaporization | | 170.8 kJ/mol (kilojoules per mole) specific heat of vaporization | | 1.276 kJ/g (kilojoules per gram) molar heat of fusion | 21.7 kJ/mol (kilojoules per mole) | 14.6 kJ/mol (kilojoules per mole) specific heat of fusion | 0.143 kJ/g (kilojoules per gram) | 0.109 kJ/g (kilojoules per gram)
Solid properties (at STP)

| cesium fluoride | lithium iodide density | 4.115 g/cm^3 | 3.49 g/cm^3 vapor pressure | 0.8 mmHg | 112 mmHg
Units
