Input interpretation
lanthanum(III) chloride hydrate | copper(I) cyanide-13C
Chemical names and formulas
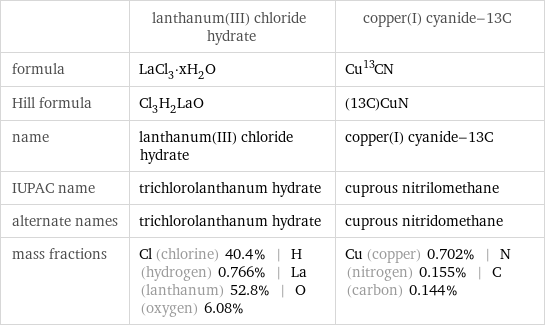
| lanthanum(III) chloride hydrate | copper(I) cyanide-13C formula | LaCl_3·xH_2O | Cu^13CN Hill formula | Cl_3H_2LaO | (13C)CuN name | lanthanum(III) chloride hydrate | copper(I) cyanide-13C IUPAC name | trichlorolanthanum hydrate | cuprous nitrilomethane alternate names | trichlorolanthanum hydrate | cuprous nitridomethane mass fractions | Cl (chlorine) 40.4% | H (hydrogen) 0.766% | La (lanthanum) 52.8% | O (oxygen) 6.08% | Cu (copper) 0.702% | N (nitrogen) 0.155% | C (carbon) 0.144%
Structure diagrams

Structure diagrams
Basic properties
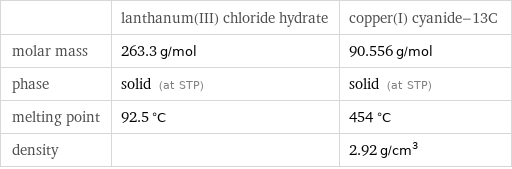
| lanthanum(III) chloride hydrate | copper(I) cyanide-13C molar mass | 263.3 g/mol | 90.556 g/mol phase | solid (at STP) | solid (at STP) melting point | 92.5 °C | 454 °C density | | 2.92 g/cm^3
Units

Solid properties (at STP)

| copper(I) cyanide-13C density | 2.92 g/cm^3
Units

Non-standard atom properties
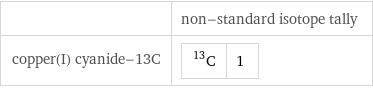
| non-standard isotope tally copper(I) cyanide-13C | C-13 | 1