Input interpretation
deuterated acetic acid (D4) | triglyme
Chemical names and formulas
![| deuterated acetic acid (D4) | triglyme formula | CD_3CO_2D | CH_3O(CH_2CH_2O)_3CH_3 Hill formula | C_2D_4O_2 | C_8H_18O_4 name | deuterated acetic acid (D4) | triglyme IUPAC name | 2, 2, 2-trideuterioacetic acid deuterio ester | 1, 2-bis(2-methoxyethoxy)ethane alternate names | 2, 2, 2-trideuterioacetic acid deuterio ester | [2H3]acetic [2H]acid | acetic acid-d4 | acetic-d3 acid-d | deuterio 2, 2, 2-trideuterioacetate | deuterio 2, 2, 2-trideuterioethanoate | tetradeuteroacetic acid | 1, 2-bis(2-methoxyethoxy)ethane | 2, 5, 8, 11-tetraoxadodecane | dimethyltriglycol | ethane, 1, 2-bis(2-methoxyethoxy)- | glyme-3 | triethylene glycol dimethyl ether mass fractions | O (oxygen) 0.49937256850014311027052826830185949802398681640625% | C (carbon) 0.374896176026952854254403746381285600364208221435546875% | H (hydrogen) 0.1257312554729040632306436009457684122025966644287109375% | C (carbon) 53.9129654150862937419% | H (hydrogen) 10.1802185964046053388% | O (oxygen) 35.9068159885091005507%](../image_source/c80601de4f1e24e60291be35ca8a37f4.png)
| deuterated acetic acid (D4) | triglyme formula | CD_3CO_2D | CH_3O(CH_2CH_2O)_3CH_3 Hill formula | C_2D_4O_2 | C_8H_18O_4 name | deuterated acetic acid (D4) | triglyme IUPAC name | 2, 2, 2-trideuterioacetic acid deuterio ester | 1, 2-bis(2-methoxyethoxy)ethane alternate names | 2, 2, 2-trideuterioacetic acid deuterio ester | [2H3]acetic [2H]acid | acetic acid-d4 | acetic-d3 acid-d | deuterio 2, 2, 2-trideuterioacetate | deuterio 2, 2, 2-trideuterioethanoate | tetradeuteroacetic acid | 1, 2-bis(2-methoxyethoxy)ethane | 2, 5, 8, 11-tetraoxadodecane | dimethyltriglycol | ethane, 1, 2-bis(2-methoxyethoxy)- | glyme-3 | triethylene glycol dimethyl ether mass fractions | O (oxygen) 0.49937256850014311027052826830185949802398681640625% | C (carbon) 0.374896176026952854254403746381285600364208221435546875% | H (hydrogen) 0.1257312554729040632306436009457684122025966644287109375% | C (carbon) 53.9129654150862937419% | H (hydrogen) 10.1802185964046053388% | O (oxygen) 35.9068159885091005507%
Structure diagrams
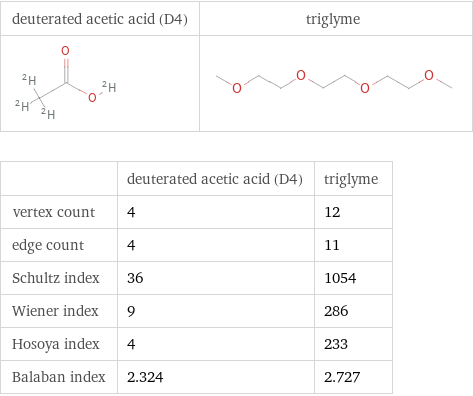
| deuterated acetic acid (D4) | triglyme vertex count | 4 | 12 edge count | 4 | 11 Schultz index | 36 | 1054 Wiener index | 9 | 286 Hosoya index | 4 | 233 Balaban index | 2.324 | 2.727
3D structure
3D structure
Basic properties
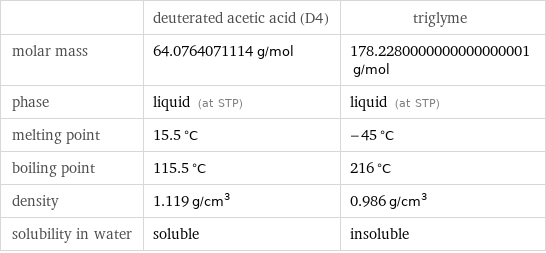
| deuterated acetic acid (D4) | triglyme molar mass | 64.0764071114 g/mol | 178.2280000000000000001 g/mol phase | liquid (at STP) | liquid (at STP) melting point | 15.5 °C | -45 °C boiling point | 115.5 °C | 216 °C density | 1.119 g/cm^3 | 0.986 g/cm^3 solubility in water | soluble | insoluble
Units

Hydrophobicity and permeability properties
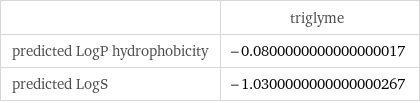
| triglyme predicted LogP hydrophobicity | -0.0800000000000000017 predicted LogS | -1.0300000000000000267
Liquid properties
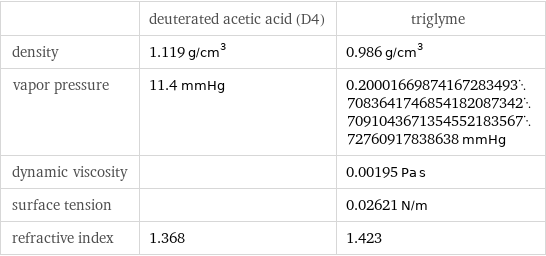
| deuterated acetic acid (D4) | triglyme density | 1.119 g/cm^3 | 0.986 g/cm^3 vapor pressure | 11.4 mmHg | 0.200016698741672834937083641746854182087342709104367135455218356772760917838638 mmHg dynamic viscosity | | 0.00195 Pa s surface tension | | 0.02621 N/m refractive index | 1.368 | 1.423
Units
Thermodynamic properties
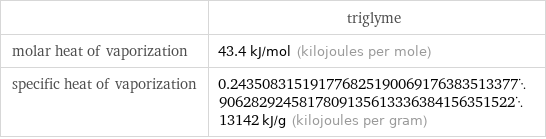
| triglyme molar heat of vaporization | 43.4 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.243508315191776825190069176383513377906282924581780913561333638415635152213142 kJ/g (kilojoules per gram)