Input interpretation
iodine monochloride | copper
Chemical names and formulas
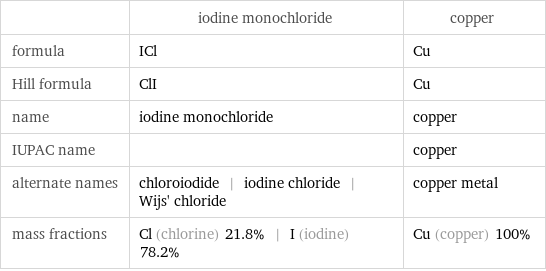
| iodine monochloride | copper formula | ICl | Cu Hill formula | ClI | Cu name | iodine monochloride | copper IUPAC name | | copper alternate names | chloroiodide | iodine chloride | Wijs' chloride | copper metal mass fractions | Cl (chlorine) 21.8% | I (iodine) 78.2% | Cu (copper) 100%
Structure diagrams

Structure diagrams
Basic properties
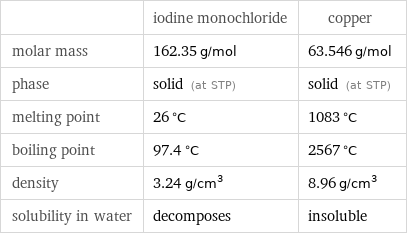
| iodine monochloride | copper molar mass | 162.35 g/mol | 63.546 g/mol phase | solid (at STP) | solid (at STP) melting point | 26 °C | 1083 °C boiling point | 97.4 °C | 2567 °C density | 3.24 g/cm^3 | 8.96 g/cm^3 solubility in water | decomposes | insoluble
Units

Thermodynamic properties
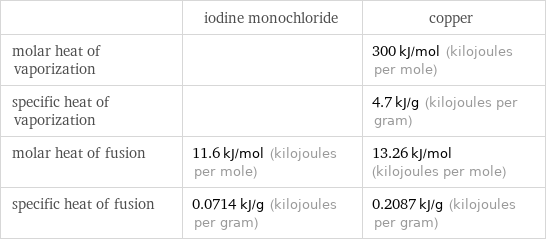
| iodine monochloride | copper molar heat of vaporization | | 300 kJ/mol (kilojoules per mole) specific heat of vaporization | | 4.7 kJ/g (kilojoules per gram) molar heat of fusion | 11.6 kJ/mol (kilojoules per mole) | 13.26 kJ/mol (kilojoules per mole) specific heat of fusion | 0.0714 kJ/g (kilojoules per gram) | 0.2087 kJ/g (kilojoules per gram)
Solid properties (at STP)

| iodine monochloride | copper density | 3.24 g/cm^3 | 8.96 g/cm^3 vapor pressure | 30 mmHg | 1 mmHg
Units
