Input interpretation
xenon | common compound names
Result
liquid xenon | xenon difluoride
Chemical names and formulas
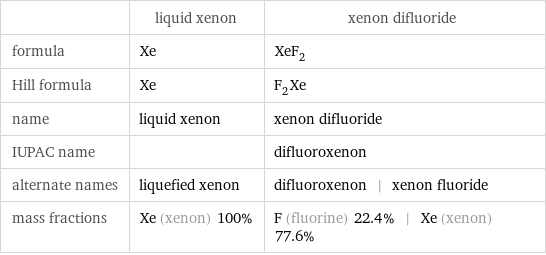
| liquid xenon | xenon difluoride formula | Xe | XeF_2 Hill formula | Xe | F_2Xe name | liquid xenon | xenon difluoride IUPAC name | | difluoroxenon alternate names | liquefied xenon | difluoroxenon | xenon fluoride mass fractions | Xe (xenon) 100% | F (fluorine) 22.4% | Xe (xenon) 77.6%
Structure diagrams

Structure diagrams
Basic properties
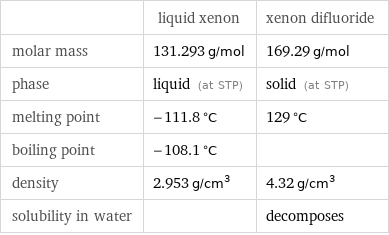
| liquid xenon | xenon difluoride molar mass | 131.293 g/mol | 169.29 g/mol phase | liquid (at STP) | solid (at STP) melting point | -111.8 °C | 129 °C boiling point | -108.1 °C | density | 2.953 g/cm^3 | 4.32 g/cm^3 solubility in water | | decomposes
Units

Liquid properties
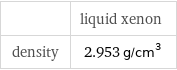
| liquid xenon density | 2.953 g/cm^3
Units

Thermodynamic properties
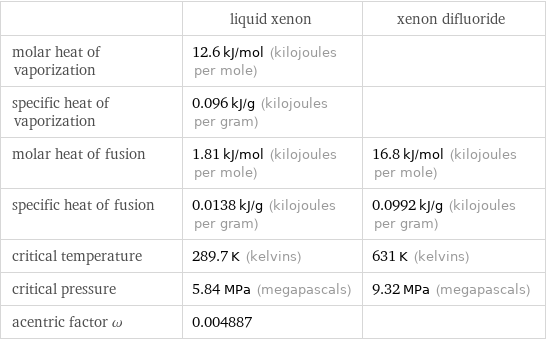
| liquid xenon | xenon difluoride molar heat of vaporization | 12.6 kJ/mol (kilojoules per mole) | specific heat of vaporization | 0.096 kJ/g (kilojoules per gram) | molar heat of fusion | 1.81 kJ/mol (kilojoules per mole) | 16.8 kJ/mol (kilojoules per mole) specific heat of fusion | 0.0138 kJ/g (kilojoules per gram) | 0.0992 kJ/g (kilojoules per gram) critical temperature | 289.7 K (kelvins) | 631 K (kelvins) critical pressure | 5.84 MPa (megapascals) | 9.32 MPa (megapascals) acentric factor ω | 0.004887 |
Solid properties
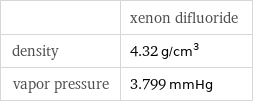
| xenon difluoride density | 4.32 g/cm^3 vapor pressure | 3.799 mmHg
Units

Chemical identifiers
![| liquid xenon | xenon difluoride CAS number | 7440-63-3 | 13709-36-9 PubChem CID number | 23991 | 83674 PubChem SID number | | 24859122 SMILES identifier | [Xe] | F[Xe]F InChI identifier | InChI=1/Xe | InChI=1/F2Xe/c1-3-2 RTECS number | | ZE1294166 MDL number | | MFCD00040538](../image_source/bddc0a359fbfc30a524005bf051ab894.png)
| liquid xenon | xenon difluoride CAS number | 7440-63-3 | 13709-36-9 PubChem CID number | 23991 | 83674 PubChem SID number | | 24859122 SMILES identifier | [Xe] | F[Xe]F InChI identifier | InChI=1/Xe | InChI=1/F2Xe/c1-3-2 RTECS number | | ZE1294166 MDL number | | MFCD00040538