Input interpretation

beryllium lithium | specific heat at STP
Results

beryllium | 1820 J/(kg K) (joules per kilogram kelvin difference) lithium | 3570 J/(kg K) (joules per kilogram kelvin difference) (value given for solid phase)
Relative values
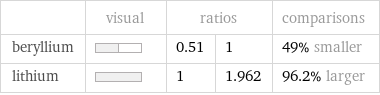
| visual | ratios | | comparisons beryllium | | 0.51 | 1 | 49% smaller lithium | | 1 | 1.962 | 96.2% larger
Thermodynamic properties
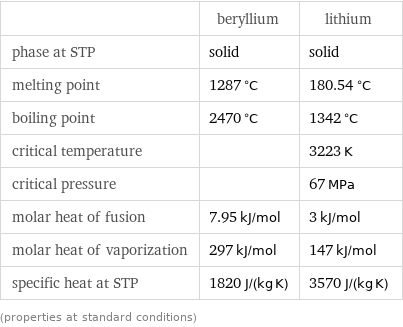
| beryllium | lithium phase at STP | solid | solid melting point | 1287 °C | 180.54 °C boiling point | 2470 °C | 1342 °C critical temperature | | 3223 K critical pressure | | 67 MPa molar heat of fusion | 7.95 kJ/mol | 3 kJ/mol molar heat of vaporization | 297 kJ/mol | 147 kJ/mol specific heat at STP | 1820 J/(kg K) | 3570 J/(kg K) (properties at standard conditions)