Input interpretation
3 mol of air
Basic properties for 3 mol

mass | 86.9 grams 0.0869 kg (kilograms) 0.192 lb (pounds) 3.06 oz (ounces) molar amount | 3 mol (moles) volume | 68.1 L (liters) 0.0681 m^3 (cubic meters) 68100 cm^3 (cubic centimeters) 144 pints 72 quarts 18 gallons 2.41 ft^3 (cubic feet) equivalents | 3 eq (equivalents) (at STP)
Thermodynamic properties for 3 mol

heat capacity C_p | gaseous | 87.45 J/K
Units

Phase change energies for 3 mol from 25 °C

energy released from cooling to condensation point | 19.2 kJ (kilojoules)
Corresponding quantities
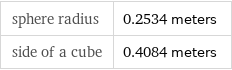
sphere radius | 0.2534 meters side of a cube | 0.4084 meters
Chemical names and formulas

name | dry air
Substance properties
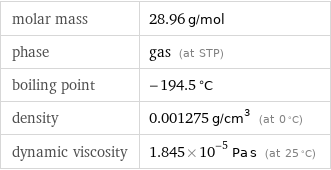
molar mass | 28.96 g/mol phase | gas (at STP) boiling point | -194.5 °C density | 0.001275 g/cm^3 (at 0 °C) dynamic viscosity | 1.845×10^-5 Pa s (at 25 °C)
Units
