Input interpretation
hydrogen chloride | hydrogen bromide
Chemical names and formulas
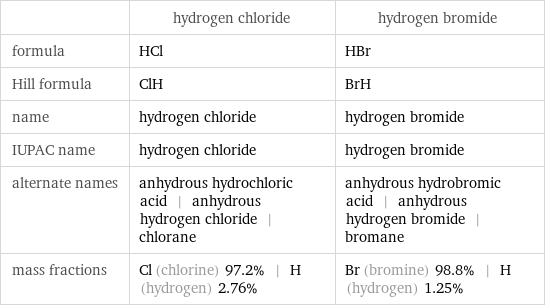
| hydrogen chloride | hydrogen bromide formula | HCl | HBr Hill formula | ClH | BrH name | hydrogen chloride | hydrogen bromide IUPAC name | hydrogen chloride | hydrogen bromide alternate names | anhydrous hydrochloric acid | anhydrous hydrogen chloride | chlorane | anhydrous hydrobromic acid | anhydrous hydrogen bromide | bromane mass fractions | Cl (chlorine) 97.2% | H (hydrogen) 2.76% | Br (bromine) 98.8% | H (hydrogen) 1.25%
Structure diagrams
| hydrogen chloride | hydrogen bromide vertex count | 1 | 1 edge count | 1 | 1 Schultz index | 0 | 0 Wiener index | 0 | 0 Hosoya index | 1 | 1 Balaban index | 0 | 0
3D structure
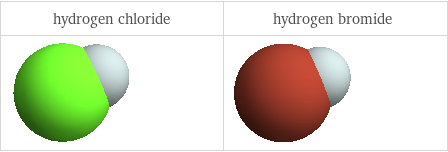
3D structure
Basic properties
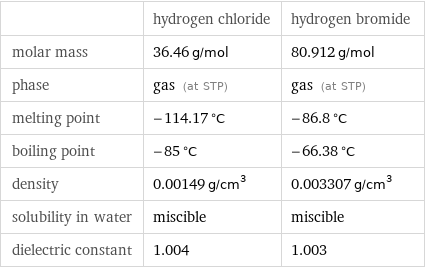
| hydrogen chloride | hydrogen bromide molar mass | 36.46 g/mol | 80.912 g/mol phase | gas (at STP) | gas (at STP) melting point | -114.17 °C | -86.8 °C boiling point | -85 °C | -66.38 °C density | 0.00149 g/cm^3 | 0.003307 g/cm^3 solubility in water | miscible | miscible dielectric constant | 1.004 | 1.003
Gas properties (at STP)
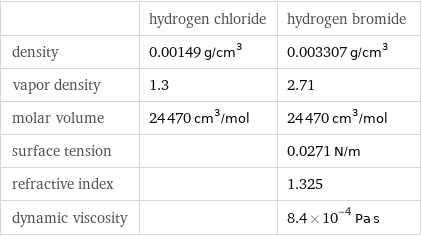
| hydrogen chloride | hydrogen bromide density | 0.00149 g/cm^3 | 0.003307 g/cm^3 vapor density | 1.3 | 2.71 molar volume | 24470 cm^3/mol | 24470 cm^3/mol surface tension | | 0.0271 N/m refractive index | | 1.325 dynamic viscosity | | 8.4×10^-4 Pa s
Units

Liquid properties

| hydrogen chloride | hydrogen bromide sound speed | 1066 km/h | 720 km/h
Units
