Input interpretation

norethindrone | orbital hybridization
Result
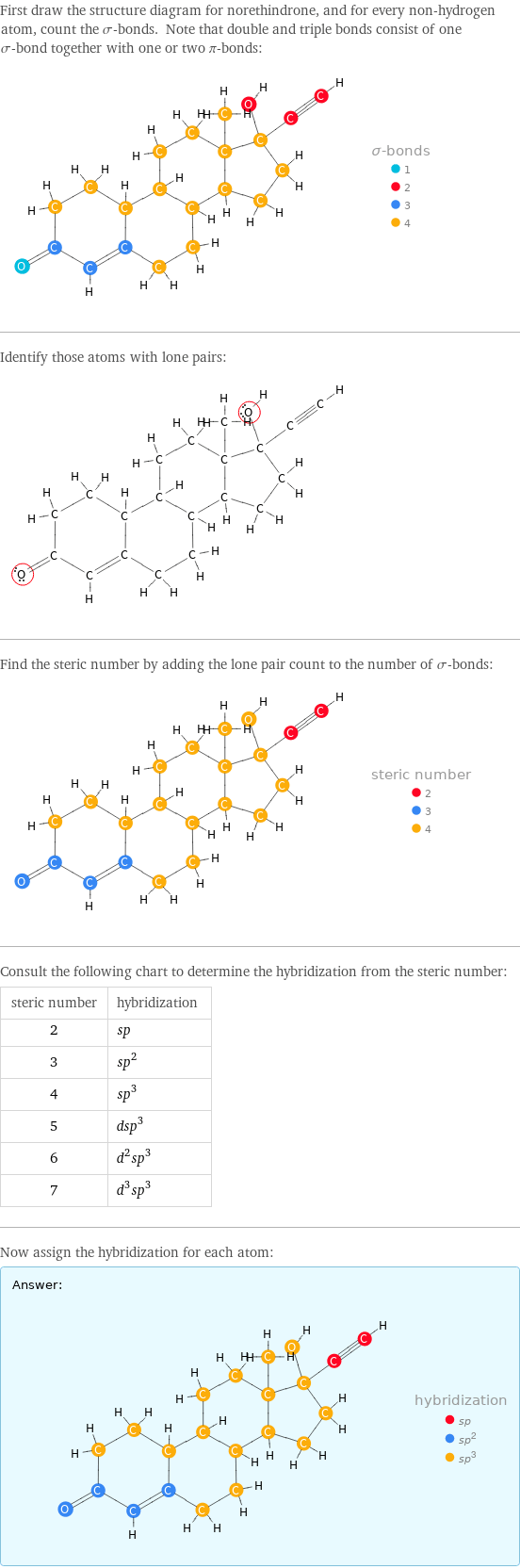
First draw the structure diagram for norethindrone, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Now assign the hybridization for each atom: Answer: | |
Chemical names and formulas
![formula | C_20H_26O_2 name | norethindrone IUPAC name | 17-ethynyl-17-hydroxy-13-methyl-1, 2, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16-dodecahydrocyclopenta[a]phenanthren-3-one alternate names | anovule | conludaf | ethinylnortestosterone | norethisterone mass fractions | C (carbon) 80.5% | H (hydrogen) 8.78% | O (oxygen) 10.7%](../image_source/4dee29309a6c31177ca3fa9a06707a9d.png)
formula | C_20H_26O_2 name | norethindrone IUPAC name | 17-ethynyl-17-hydroxy-13-methyl-1, 2, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16-dodecahydrocyclopenta[a]phenanthren-3-one alternate names | anovule | conludaf | ethinylnortestosterone | norethisterone mass fractions | C (carbon) 80.5% | H (hydrogen) 8.78% | O (oxygen) 10.7%