Input interpretation
lithium hydroxide | perchloric acid
Chemical names and formulas
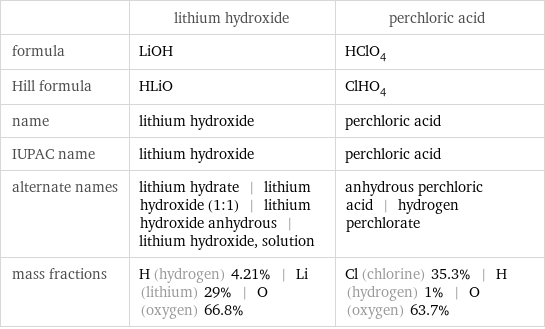
| lithium hydroxide | perchloric acid formula | LiOH | HClO_4 Hill formula | HLiO | ClHO_4 name | lithium hydroxide | perchloric acid IUPAC name | lithium hydroxide | perchloric acid alternate names | lithium hydrate | lithium hydroxide (1:1) | lithium hydroxide anhydrous | lithium hydroxide, solution | anhydrous perchloric acid | hydrogen perchlorate mass fractions | H (hydrogen) 4.21% | Li (lithium) 29% | O (oxygen) 66.8% | Cl (chlorine) 35.3% | H (hydrogen) 1% | O (oxygen) 63.7%
Structure diagrams
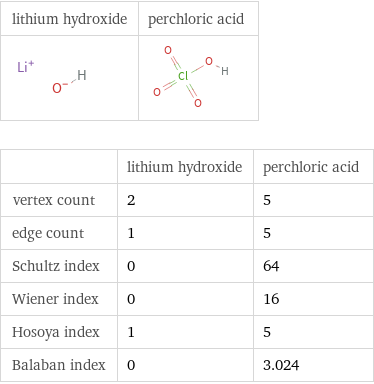
| lithium hydroxide | perchloric acid vertex count | 2 | 5 edge count | 1 | 5 Schultz index | 0 | 64 Wiener index | 0 | 16 Hosoya index | 1 | 5 Balaban index | 0 | 3.024
Basic properties
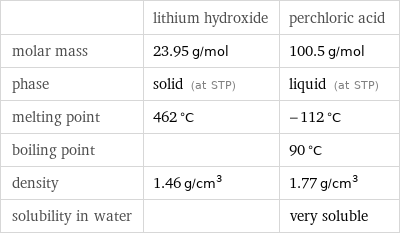
| lithium hydroxide | perchloric acid molar mass | 23.95 g/mol | 100.5 g/mol phase | solid (at STP) | liquid (at STP) melting point | 462 °C | -112 °C boiling point | | 90 °C density | 1.46 g/cm^3 | 1.77 g/cm^3 solubility in water | | very soluble
Units

Liquid properties
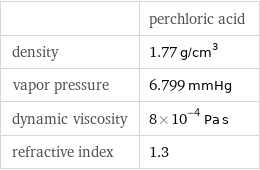
| perchloric acid density | 1.77 g/cm^3 vapor pressure | 6.799 mmHg dynamic viscosity | 8×10^-4 Pa s refractive index | 1.3
Units
Thermodynamic properties
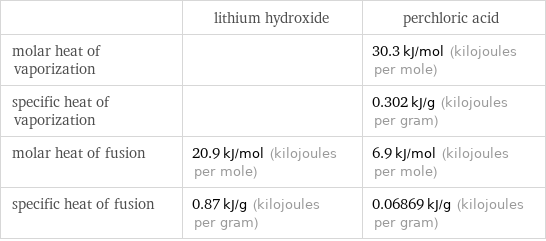
| lithium hydroxide | perchloric acid molar heat of vaporization | | 30.3 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.302 kJ/g (kilojoules per gram) molar heat of fusion | 20.9 kJ/mol (kilojoules per mole) | 6.9 kJ/mol (kilojoules per mole) specific heat of fusion | 0.87 kJ/g (kilojoules per gram) | 0.06869 kJ/g (kilojoules per gram)
Solid properties

| lithium hydroxide density | 1.46 g/cm^3 vapor pressure | 2 mmHg
Units
