Input interpretation
silver chloride | rubidium fluoride
Chemical names and formulas
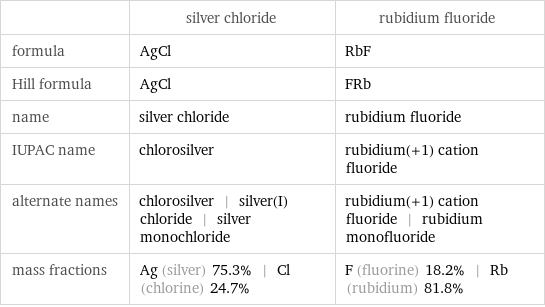
| silver chloride | rubidium fluoride formula | AgCl | RbF Hill formula | AgCl | FRb name | silver chloride | rubidium fluoride IUPAC name | chlorosilver | rubidium(+1) cation fluoride alternate names | chlorosilver | silver(I) chloride | silver monochloride | rubidium(+1) cation fluoride | rubidium monofluoride mass fractions | Ag (silver) 75.3% | Cl (chlorine) 24.7% | F (fluorine) 18.2% | Rb (rubidium) 81.8%
Structure diagrams

Structure diagrams
Basic properties
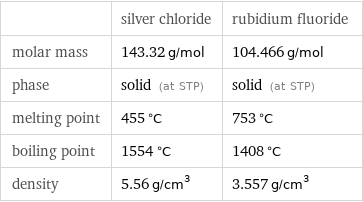
| silver chloride | rubidium fluoride molar mass | 143.32 g/mol | 104.466 g/mol phase | solid (at STP) | solid (at STP) melting point | 455 °C | 753 °C boiling point | 1554 °C | 1408 °C density | 5.56 g/cm^3 | 3.557 g/cm^3
Units

Thermodynamic properties
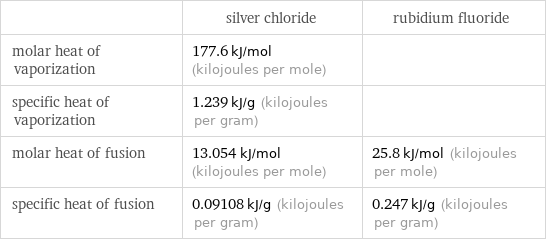
| silver chloride | rubidium fluoride molar heat of vaporization | 177.6 kJ/mol (kilojoules per mole) | specific heat of vaporization | 1.239 kJ/g (kilojoules per gram) | molar heat of fusion | 13.054 kJ/mol (kilojoules per mole) | 25.8 kJ/mol (kilojoules per mole) specific heat of fusion | 0.09108 kJ/g (kilojoules per gram) | 0.247 kJ/g (kilojoules per gram)