Input interpretation

barium permanganate | molar mass
Result
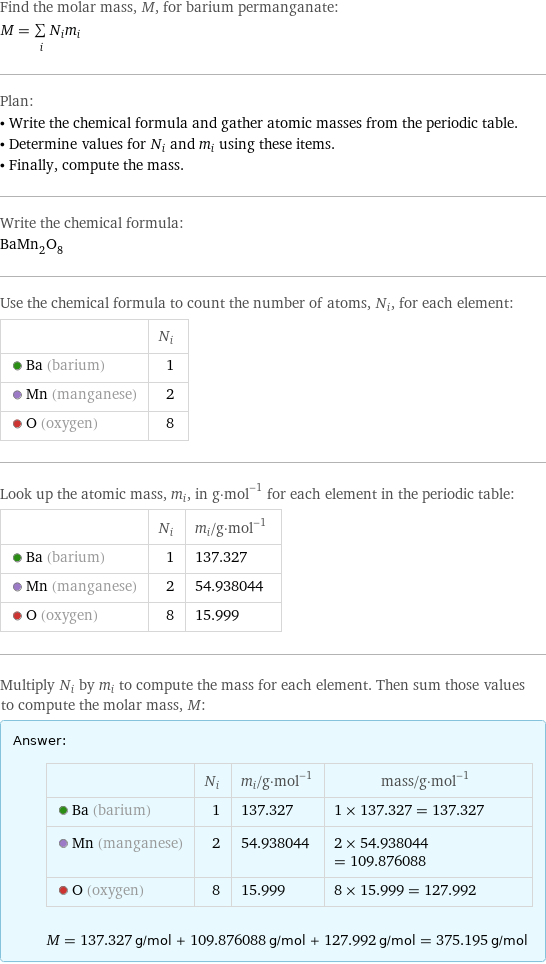
Find the molar mass, M, for barium permanganate: M = sum _iN_im_i Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i and m_i using these items. • Finally, compute the mass. Write the chemical formula: BaMn_2O_8 Use the chemical formula to count the number of atoms, N_i, for each element: | N_i Ba (barium) | 1 Mn (manganese) | 2 O (oxygen) | 8 Look up the atomic mass, m_i, in g·mol^(-1) for each element in the periodic table: | N_i | m_i/g·mol^(-1) Ba (barium) | 1 | 137.327 Mn (manganese) | 2 | 54.938044 O (oxygen) | 8 | 15.999 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molar mass, M: Answer: | | | N_i | m_i/g·mol^(-1) | mass/g·mol^(-1) Ba (barium) | 1 | 137.327 | 1 × 137.327 = 137.327 Mn (manganese) | 2 | 54.938044 | 2 × 54.938044 = 109.876088 O (oxygen) | 8 | 15.999 | 8 × 15.999 = 127.992 M = 137.327 g/mol + 109.876088 g/mol + 127.992 g/mol = 375.195 g/mol
Unit conversion
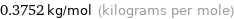
0.3752 kg/mol (kilograms per mole)
Comparisons
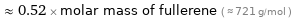
≈ 0.52 × molar mass of fullerene ( ≈ 721 g/mol )
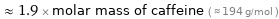
≈ 1.9 × molar mass of caffeine ( ≈ 194 g/mol )
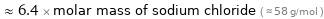
≈ 6.4 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
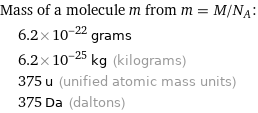
Mass of a molecule m from m = M/N_A: | 6.2×10^-22 grams | 6.2×10^-25 kg (kilograms) | 375 u (unified atomic mass units) | 375 Da (daltons)
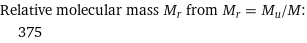
Relative molecular mass M_r from M_r = M_u/M: | 375