Input interpretation

trans-diamminedichloropalladium(II) | element types
Result
chlorine | hydrogen | nitrogen | palladium
Periodic table location
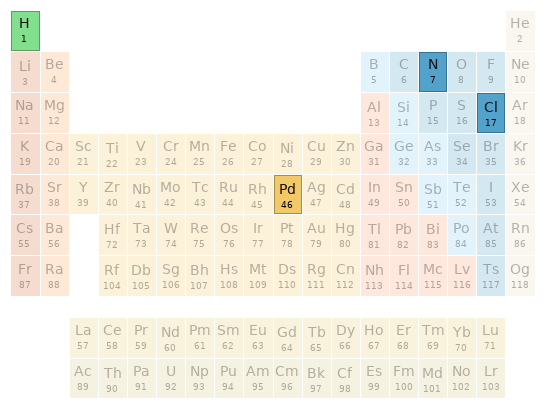
Periodic table location
Images
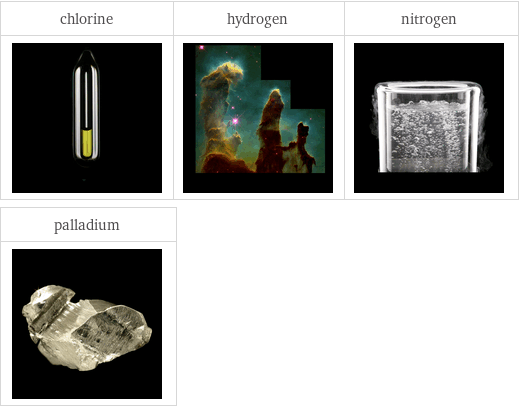
Images
Basic elemental properties
![| atomic symbol | atomic number | short electronic configuration chlorine | Cl | 17 | [Ne]3s^23p^5 hydrogen | H | 1 | 1s^1 nitrogen | N | 7 | [He]2s^22p^3 palladium | Pd | 46 | [Kr]4d^10 | Aufbau diagram | block | group chlorine | 3p 3s | p | 17 hydrogen | 1s | s | 1 nitrogen | 2p 2s | p | 15 palladium | 4d | d | 10 | period | atomic mass | half-life chlorine | 3 | 35.45 u | (stable) hydrogen | 1 | 1.008 u | (stable) nitrogen | 2 | 14.007 u | (stable) palladium | 5 | 106.42 u | (stable)](../image_source/cee816f94126033053f9d732097ffd77.png)
| atomic symbol | atomic number | short electronic configuration chlorine | Cl | 17 | [Ne]3s^23p^5 hydrogen | H | 1 | 1s^1 nitrogen | N | 7 | [He]2s^22p^3 palladium | Pd | 46 | [Kr]4d^10 | Aufbau diagram | block | group chlorine | 3p 3s | p | 17 hydrogen | 1s | s | 1 nitrogen | 2p 2s | p | 15 palladium | 4d | d | 10 | period | atomic mass | half-life chlorine | 3 | 35.45 u | (stable) hydrogen | 1 | 1.008 u | (stable) nitrogen | 2 | 14.007 u | (stable) palladium | 5 | 106.42 u | (stable)
Thermodynamic properties
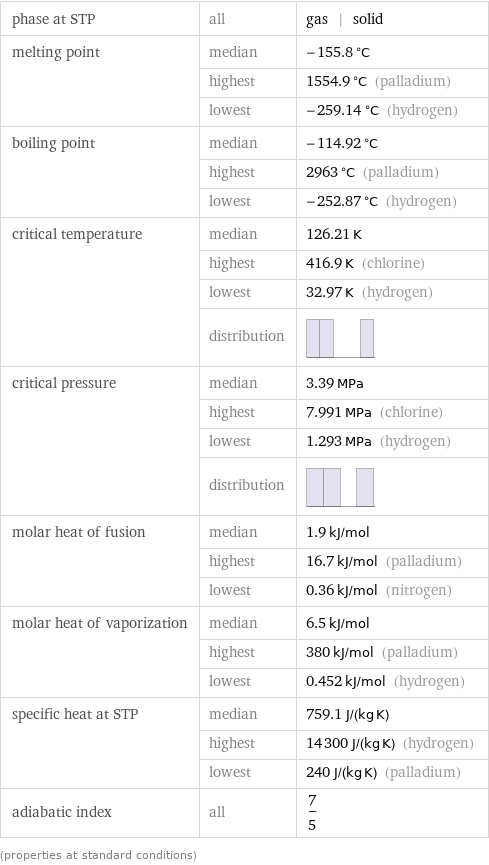
phase at STP | all | gas | solid melting point | median | -155.8 °C | highest | 1554.9 °C (palladium) | lowest | -259.14 °C (hydrogen) boiling point | median | -114.92 °C | highest | 2963 °C (palladium) | lowest | -252.87 °C (hydrogen) critical temperature | median | 126.21 K | highest | 416.9 K (chlorine) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 3.39 MPa | highest | 7.991 MPa (chlorine) | lowest | 1.293 MPa (hydrogen) | distribution | molar heat of fusion | median | 1.9 kJ/mol | highest | 16.7 kJ/mol (palladium) | lowest | 0.36 kJ/mol (nitrogen) molar heat of vaporization | median | 6.5 kJ/mol | highest | 380 kJ/mol (palladium) | lowest | 0.452 kJ/mol (hydrogen) specific heat at STP | median | 759.1 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 240 J/(kg K) (palladium) adiabatic index | all | 7/5 (properties at standard conditions)
Material properties
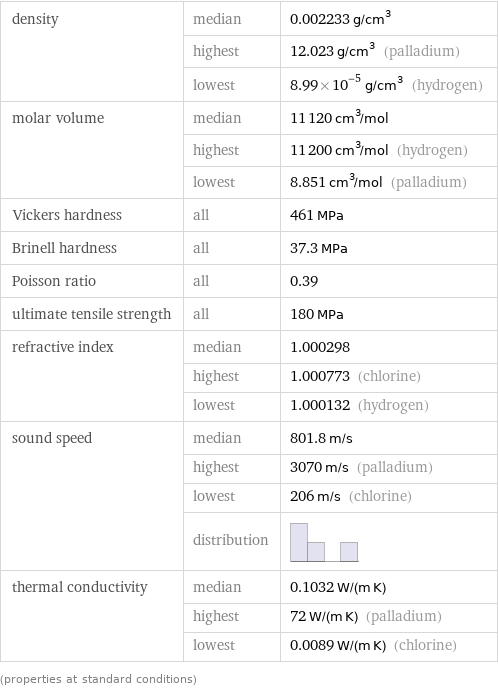
density | median | 0.002233 g/cm^3 | highest | 12.023 g/cm^3 (palladium) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) molar volume | median | 11120 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 8.851 cm^3/mol (palladium) Vickers hardness | all | 461 MPa Brinell hardness | all | 37.3 MPa Poisson ratio | all | 0.39 ultimate tensile strength | all | 180 MPa refractive index | median | 1.000298 | highest | 1.000773 (chlorine) | lowest | 1.000132 (hydrogen) sound speed | median | 801.8 m/s | highest | 3070 m/s (palladium) | lowest | 206 m/s (chlorine) | distribution | thermal conductivity | median | 0.1032 W/(m K) | highest | 72 W/(m K) (palladium) | lowest | 0.0089 W/(m K) (chlorine) (properties at standard conditions)
Reactivity
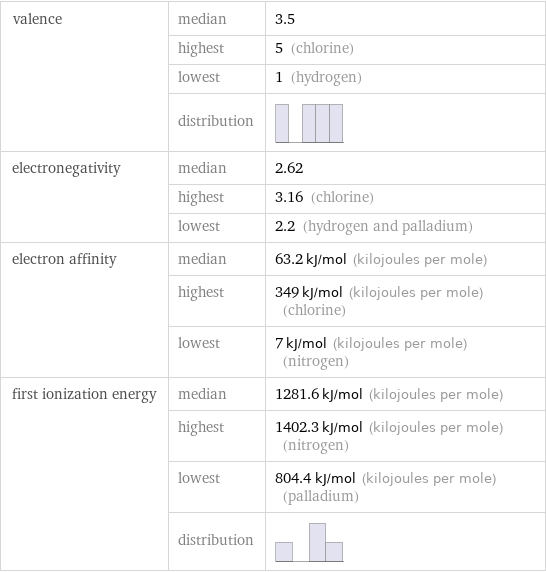
valence | median | 3.5 | highest | 5 (chlorine) | lowest | 1 (hydrogen) | distribution | electronegativity | median | 2.62 | highest | 3.16 (chlorine) | lowest | 2.2 (hydrogen and palladium) electron affinity | median | 63.2 kJ/mol (kilojoules per mole) | highest | 349 kJ/mol (kilojoules per mole) (chlorine) | lowest | 7 kJ/mol (kilojoules per mole) (nitrogen) first ionization energy | median | 1281.6 kJ/mol (kilojoules per mole) | highest | 1402.3 kJ/mol (kilojoules per mole) (nitrogen) | lowest | 804.4 kJ/mol (kilojoules per mole) (palladium) | distribution |
Atomic properties
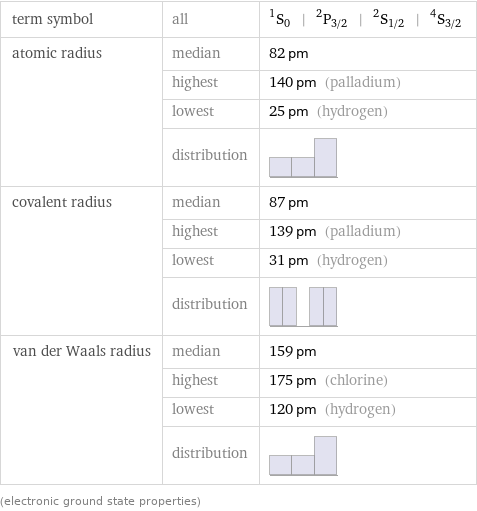
term symbol | all | ^1S_0 | ^2P_(3/2) | ^2S_(1/2) | ^4S_(3/2) atomic radius | median | 82 pm | highest | 140 pm (palladium) | lowest | 25 pm (hydrogen) | distribution | covalent radius | median | 87 pm | highest | 139 pm (palladium) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 159 pm | highest | 175 pm (chlorine) | lowest | 120 pm (hydrogen) | distribution | (electronic ground state properties)
Abundances
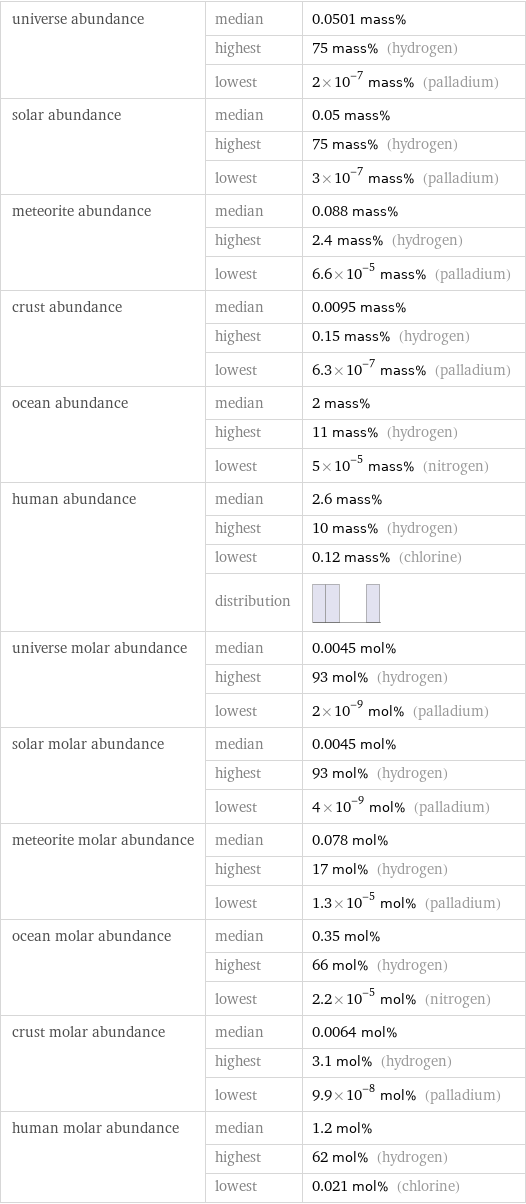
universe abundance | median | 0.0501 mass% | highest | 75 mass% (hydrogen) | lowest | 2×10^-7 mass% (palladium) solar abundance | median | 0.05 mass% | highest | 75 mass% (hydrogen) | lowest | 3×10^-7 mass% (palladium) meteorite abundance | median | 0.088 mass% | highest | 2.4 mass% (hydrogen) | lowest | 6.6×10^-5 mass% (palladium) crust abundance | median | 0.0095 mass% | highest | 0.15 mass% (hydrogen) | lowest | 6.3×10^-7 mass% (palladium) ocean abundance | median | 2 mass% | highest | 11 mass% (hydrogen) | lowest | 5×10^-5 mass% (nitrogen) human abundance | median | 2.6 mass% | highest | 10 mass% (hydrogen) | lowest | 0.12 mass% (chlorine) | distribution | universe molar abundance | median | 0.0045 mol% | highest | 93 mol% (hydrogen) | lowest | 2×10^-9 mol% (palladium) solar molar abundance | median | 0.0045 mol% | highest | 93 mol% (hydrogen) | lowest | 4×10^-9 mol% (palladium) meteorite molar abundance | median | 0.078 mol% | highest | 17 mol% (hydrogen) | lowest | 1.3×10^-5 mol% (palladium) ocean molar abundance | median | 0.35 mol% | highest | 66 mol% (hydrogen) | lowest | 2.2×10^-5 mol% (nitrogen) crust molar abundance | median | 0.0064 mol% | highest | 3.1 mol% (hydrogen) | lowest | 9.9×10^-8 mol% (palladium) human molar abundance | median | 1.2 mol% | highest | 62 mol% (hydrogen) | lowest | 0.021 mol% (chlorine)