Input interpretation
aluminum fluoride
Chemical names and formulas
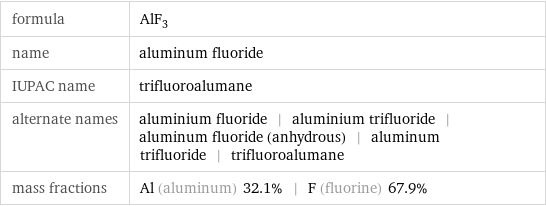
formula | AlF_3 name | aluminum fluoride IUPAC name | trifluoroalumane alternate names | aluminium fluoride | aluminium trifluoride | aluminum fluoride (anhydrous) | aluminum trifluoride | trifluoroalumane mass fractions | Al (aluminum) 32.1% | F (fluorine) 67.9%
Lewis structure

Draw the Lewis structure of aluminum fluoride. Start by drawing the overall structure of the molecule: Count the total valence electrons of the aluminum (n_Al, val = 3) and fluorine (n_F, val = 7) atoms: n_Al, val + 3 n_F, val = 24 Calculate the number of electrons needed to completely fill the valence shells for aluminum (n_Al, full = 6) and fluorine (n_F, full = 8): n_Al, full + 3 n_F, full = 30 Subtracting these two numbers shows that 30 - 24 = 6 bonding electrons are needed. Each bond has two electrons, so the above diagram has all the necessary bonds. There are 3 bonds and hence 6 bonding electrons in the diagram. Lastly, fill in the remaining unbonded electrons on each atom. In total, there remain 24 - 6 = 18 electrons left to draw: Answer: | |
Basic properties
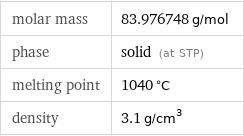
molar mass | 83.976748 g/mol phase | solid (at STP) melting point | 1040 °C density | 3.1 g/cm^3
Units

Solid properties (at STP)
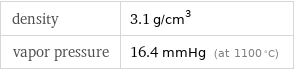
density | 3.1 g/cm^3 vapor pressure | 16.4 mmHg (at 1100 °C)
Units

Thermodynamic properties
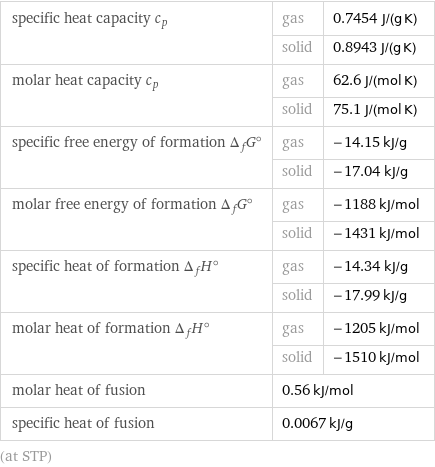
specific heat capacity c_p | gas | 0.7454 J/(g K) | solid | 0.8943 J/(g K) molar heat capacity c_p | gas | 62.6 J/(mol K) | solid | 75.1 J/(mol K) specific free energy of formation Δ_fG° | gas | -14.15 kJ/g | solid | -17.04 kJ/g molar free energy of formation Δ_fG° | gas | -1188 kJ/mol | solid | -1431 kJ/mol specific heat of formation Δ_fH° | gas | -14.34 kJ/g | solid | -17.99 kJ/g molar heat of formation Δ_fH° | gas | -1205 kJ/mol | solid | -1510 kJ/mol molar heat of fusion | 0.56 kJ/mol | specific heat of fusion | 0.0067 kJ/g | (at STP)
Chemical identifiers
F InChI identifier | InChI=1/Al.3FH/h;3*1H/q+3;;;/p-3/fAl.3F/h;3*1h/qm;3*-1 RTECS number | BD0725000 MDL number | MFCD00003426](../image_source/15f9aa88b69d552dbd028099bfde036f.png)
CAS number | 7784-18-1 PubChem CID number | 2124 PubChem SID number | 24860089 SMILES identifier | F[Al](F)F InChI identifier | InChI=1/Al.3FH/h;3*1H/q+3;;;/p-3/fAl.3F/h;3*1h/qm;3*-1 RTECS number | BD0725000 MDL number | MFCD00003426
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | primary irritant