Input interpretation
sulfamic acid | lead(II) hydroxide
Chemical names and formulas
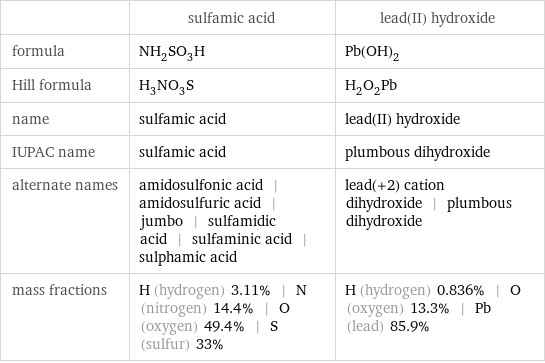
| sulfamic acid | lead(II) hydroxide formula | NH_2SO_3H | Pb(OH)_2 Hill formula | H_3NO_3S | H_2O_2Pb name | sulfamic acid | lead(II) hydroxide IUPAC name | sulfamic acid | plumbous dihydroxide alternate names | amidosulfonic acid | amidosulfuric acid | jumbo | sulfamidic acid | sulfaminic acid | sulphamic acid | lead(+2) cation dihydroxide | plumbous dihydroxide mass fractions | H (hydrogen) 3.11% | N (nitrogen) 14.4% | O (oxygen) 49.4% | S (sulfur) 33% | H (hydrogen) 0.836% | O (oxygen) 13.3% | Pb (lead) 85.9%
Structure diagrams
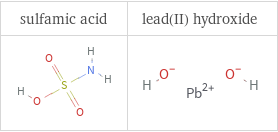
Structure diagrams
3D structure
3D structure
Basic properties
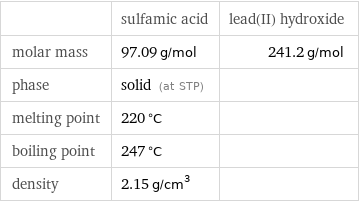
| sulfamic acid | lead(II) hydroxide molar mass | 97.09 g/mol | 241.2 g/mol phase | solid (at STP) | melting point | 220 °C | boiling point | 247 °C | density | 2.15 g/cm^3 |
Units

Solid properties
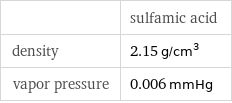
| sulfamic acid density | 2.15 g/cm^3 vapor pressure | 0.006 mmHg
Units
