Input interpretation

4-methoxytriphenylamine | aromatic atom count
Result
18 atoms
Aromatic atoms in place
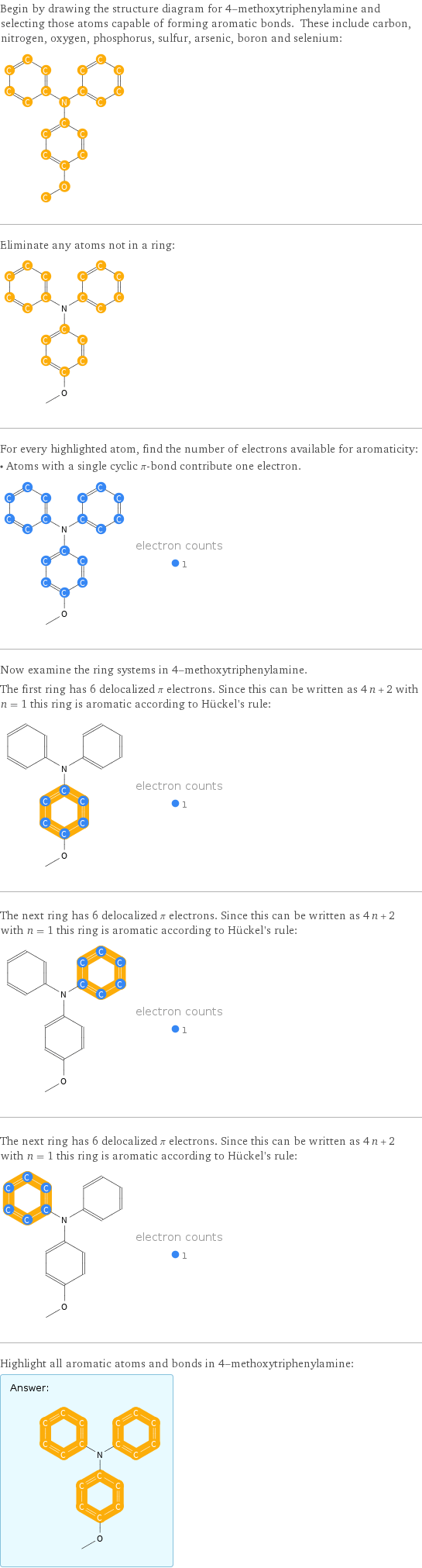
Begin by drawing the structure diagram for 4-methoxytriphenylamine and selecting those atoms capable of forming aromatic bonds. These include carbon, nitrogen, oxygen, phosphorus, sulfur, arsenic, boron and selenium: Eliminate any atoms not in a ring: For every highlighted atom, find the number of electrons available for aromaticity: • Atoms with a single cyclic π-bond contribute one electron. Now examine the ring systems in 4-methoxytriphenylamine. The first ring has 6 delocalized π electrons. Since this can be written as 4 n + 2 with n = 1 this ring is aromatic according to Hückel's rule: The next ring has 6 delocalized π electrons. Since this can be written as 4 n + 2 with n = 1 this ring is aromatic according to Hückel's rule: The next ring has 6 delocalized π electrons. Since this can be written as 4 n + 2 with n = 1 this ring is aromatic according to Hückel's rule: Highlight all aromatic atoms and bonds in 4-methoxytriphenylamine: Answer: | |
Aromatic atoms elemental composition
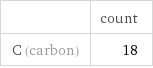
| count C (carbon) | 18
Quantitative molecular descriptors
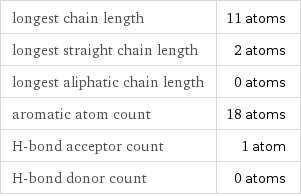
longest chain length | 11 atoms longest straight chain length | 2 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 18 atoms H-bond acceptor count | 1 atom H-bond donor count | 0 atoms
Corresponding quantity

Moles of atoms from n = N/N_A: | 3×10^-23 mol (moles)