Input interpretation
halogenated compounds | electron count
Summary
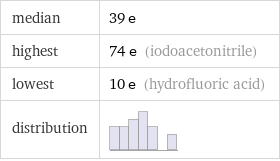
median | 39 e highest | 74 e (iodoacetonitrile) lowest | 10 e (hydrofluoric acid) distribution |
Units

Distribution plots
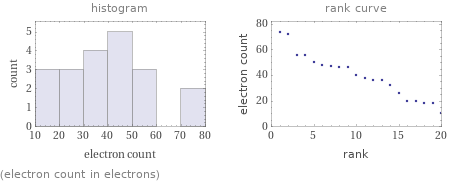
(electron count in electrons)
Electron count rankings
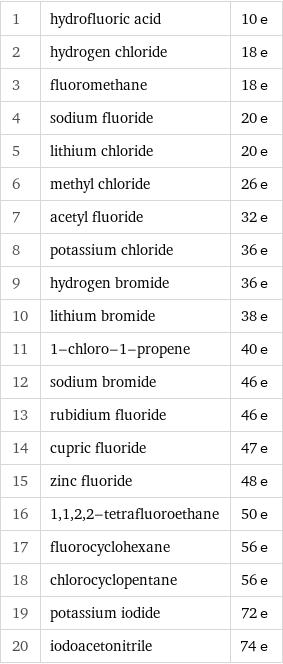
1 | hydrofluoric acid | 10 e 2 | hydrogen chloride | 18 e 3 | fluoromethane | 18 e 4 | sodium fluoride | 20 e 5 | lithium chloride | 20 e 6 | methyl chloride | 26 e 7 | acetyl fluoride | 32 e 8 | potassium chloride | 36 e 9 | hydrogen bromide | 36 e 10 | lithium bromide | 38 e 11 | 1-chloro-1-propene | 40 e 12 | sodium bromide | 46 e 13 | rubidium fluoride | 46 e 14 | cupric fluoride | 47 e 15 | zinc fluoride | 48 e 16 | 1, 1, 2, 2-tetrafluoroethane | 50 e 17 | fluorocyclohexane | 56 e 18 | chlorocyclopentane | 56 e 19 | potassium iodide | 72 e 20 | iodoacetonitrile | 74 e
Corresponding quantity
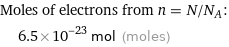
Moles of electrons from n = N/N_A: | 6.5×10^-23 mol (moles)