Input interpretation
trisilane | iron(II) aluminate
Chemical names and formulas
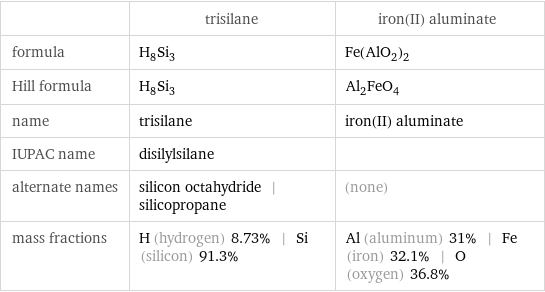
| trisilane | iron(II) aluminate formula | H_8Si_3 | Fe(AlO_2)_2 Hill formula | H_8Si_3 | Al_2FeO_4 name | trisilane | iron(II) aluminate IUPAC name | disilylsilane | alternate names | silicon octahydride | silicopropane | (none) mass fractions | H (hydrogen) 8.73% | Si (silicon) 91.3% | Al (aluminum) 31% | Fe (iron) 32.1% | O (oxygen) 36.8%
Structure diagrams
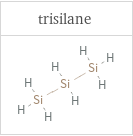
Structure diagrams
3D structure
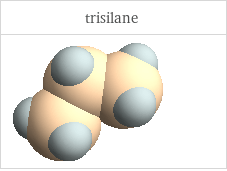
3D structure
Basic properties
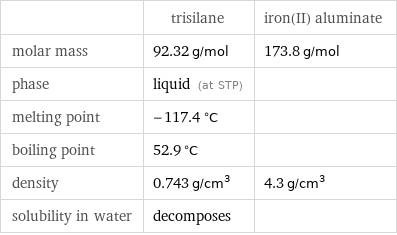
| trisilane | iron(II) aluminate molar mass | 92.32 g/mol | 173.8 g/mol phase | liquid (at STP) | melting point | -117.4 °C | boiling point | 52.9 °C | density | 0.743 g/cm^3 | 4.3 g/cm^3 solubility in water | decomposes |
Units
