Input interpretation
soda ash | magnesium hydroxide
Chemical names and formulas
![| soda ash | magnesium hydroxide formula | Na_2CO_3 | Mg(OH)_2 Hill formula | CNa_2O_3 | H_2MgO_2 name | soda ash | magnesium hydroxide IUPAC name | disodium carbonate | magnesium dihydroxide alternate names | crystol carbonate | disodium carbonate | soda | sodium carbonate | magnesia magma | magnesia, [Milk of] | magnesium dihydroxide | magnesium hydrate | milk of magnesia mass fractions | C (carbon) 11.3% | Na (sodium) 43.4% | O (oxygen) 45.3% | H (hydrogen) 3.46% | Mg (magnesium) 41.7% | O (oxygen) 54.9%](../image_source/f7ac68168f8607348ccfb119c3ea38e4.png)
| soda ash | magnesium hydroxide formula | Na_2CO_3 | Mg(OH)_2 Hill formula | CNa_2O_3 | H_2MgO_2 name | soda ash | magnesium hydroxide IUPAC name | disodium carbonate | magnesium dihydroxide alternate names | crystol carbonate | disodium carbonate | soda | sodium carbonate | magnesia magma | magnesia, [Milk of] | magnesium dihydroxide | magnesium hydrate | milk of magnesia mass fractions | C (carbon) 11.3% | Na (sodium) 43.4% | O (oxygen) 45.3% | H (hydrogen) 3.46% | Mg (magnesium) 41.7% | O (oxygen) 54.9%
Structure diagrams
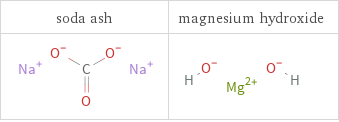
Structure diagrams
Basic properties
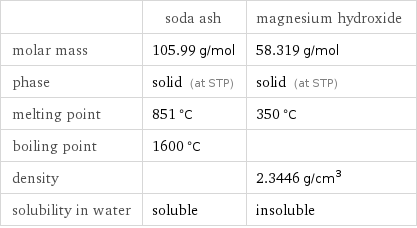
| soda ash | magnesium hydroxide molar mass | 105.99 g/mol | 58.319 g/mol phase | solid (at STP) | solid (at STP) melting point | 851 °C | 350 °C boiling point | 1600 °C | density | | 2.3446 g/cm^3 solubility in water | soluble | insoluble
Units
