Input interpretation
bismuth sulfide | lead
Chemical names and formulas
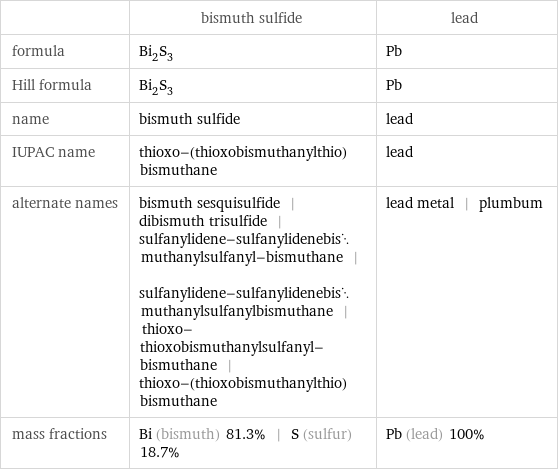
| bismuth sulfide | lead formula | Bi_2S_3 | Pb Hill formula | Bi_2S_3 | Pb name | bismuth sulfide | lead IUPAC name | thioxo-(thioxobismuthanylthio)bismuthane | lead alternate names | bismuth sesquisulfide | dibismuth trisulfide | sulfanylidene-sulfanylidenebismuthanylsulfanyl-bismuthane | sulfanylidene-sulfanylidenebismuthanylsulfanylbismuthane | thioxo-thioxobismuthanylsulfanyl-bismuthane | thioxo-(thioxobismuthanylthio)bismuthane | lead metal | plumbum mass fractions | Bi (bismuth) 81.3% | S (sulfur) 18.7% | Pb (lead) 100%
Structure diagrams
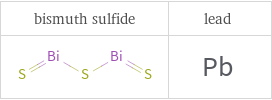
Structure diagrams
Basic properties
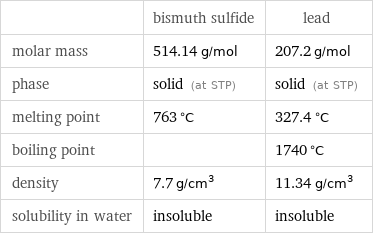
| bismuth sulfide | lead molar mass | 514.14 g/mol | 207.2 g/mol phase | solid (at STP) | solid (at STP) melting point | 763 °C | 327.4 °C boiling point | | 1740 °C density | 7.7 g/cm^3 | 11.34 g/cm^3 solubility in water | insoluble | insoluble
Units

Thermodynamic properties
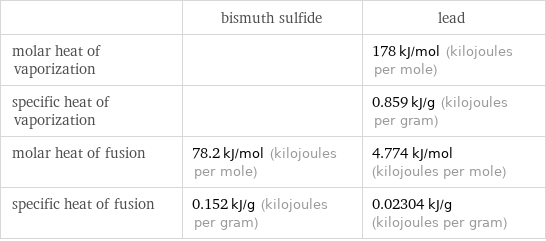
| bismuth sulfide | lead molar heat of vaporization | | 178 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.859 kJ/g (kilojoules per gram) molar heat of fusion | 78.2 kJ/mol (kilojoules per mole) | 4.774 kJ/mol (kilojoules per mole) specific heat of fusion | 0.152 kJ/g (kilojoules per gram) | 0.02304 kJ/g (kilojoules per gram)
Solid properties (at STP)

| bismuth sulfide | lead density | 7.7 g/cm^3 | 11.34 g/cm^3 vapor pressure | | 5×10^-4 mmHg
Units

Chemical identifiers
![| bismuth sulfide | lead CAS number | 1345-07-9 | 7439-92-1 PubChem CID number | 16682960 | 5352425 PubChem SID number | 24860111 | 24852588 SMILES identifier | S=[Bi]S[Bi]=S | [Pb]](../image_source/e44fde136b4b84efce93356b71d0f3c6.png)
| bismuth sulfide | lead CAS number | 1345-07-9 | 7439-92-1 PubChem CID number | 16682960 | 5352425 PubChem SID number | 24860111 | 24852588 SMILES identifier | S=[Bi]S[Bi]=S | [Pb]
NFPA label

NFPA label