Input interpretation

organic solvents | Henry law constant
Summary
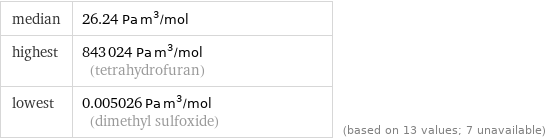
median | 26.24 Pa m^3/mol highest | 843024 Pa m^3/mol (tetrahydrofuran) lowest | 0.005026 Pa m^3/mol (dimethyl sulfoxide) | (based on 13 values; 7 unavailable)
Entities with missing values

acetonitrile | chlorobenzene | cyclohexane | ethylene dichloride | hexamethylphosphoric triamide | nitromethane | pyridine (total: 7)
Distribution plots
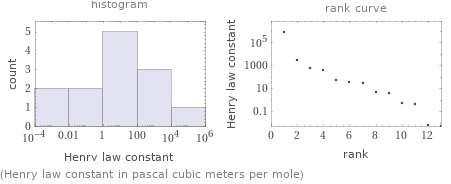
(Henry law constant in pascal cubic meters per mole)
Henry law constant rankings
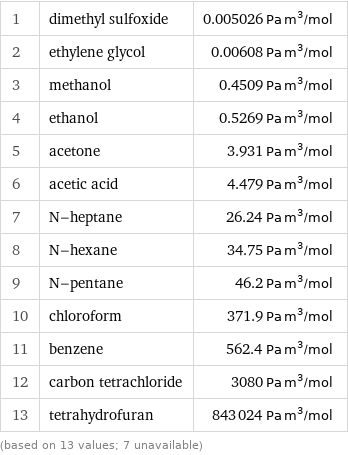
1 | dimethyl sulfoxide | 0.005026 Pa m^3/mol 2 | ethylene glycol | 0.00608 Pa m^3/mol 3 | methanol | 0.4509 Pa m^3/mol 4 | ethanol | 0.5269 Pa m^3/mol 5 | acetone | 3.931 Pa m^3/mol 6 | acetic acid | 4.479 Pa m^3/mol 7 | N-heptane | 26.24 Pa m^3/mol 8 | N-hexane | 34.75 Pa m^3/mol 9 | N-pentane | 46.2 Pa m^3/mol 10 | chloroform | 371.9 Pa m^3/mol 11 | benzene | 562.4 Pa m^3/mol 12 | carbon tetrachloride | 3080 Pa m^3/mol 13 | tetrahydrofuran | 843024 Pa m^3/mol (based on 13 values; 7 unavailable)
Unit conversion for median Henry law constant 26.24 Pa m^3/mol

0.259 atm L/mol (atmosphere liters per mole)