Input interpretation
beryllium
Chemical names and formulas
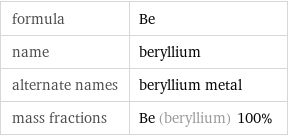
formula | Be name | beryllium alternate names | beryllium metal mass fractions | Be (beryllium) 100%
Structure diagram

Structure diagram
Basic properties
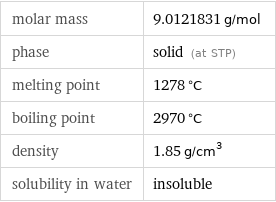
molar mass | 9.0121831 g/mol phase | solid (at STP) melting point | 1278 °C boiling point | 2970 °C density | 1.85 g/cm^3 solubility in water | insoluble
Units

Solid properties (at STP)

density | 1.85 g/cm^3
Units

Thermodynamic properties
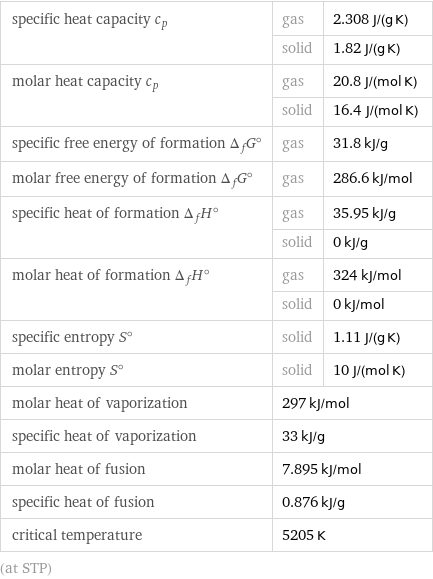
specific heat capacity c_p | gas | 2.308 J/(g K) | solid | 1.82 J/(g K) molar heat capacity c_p | gas | 20.8 J/(mol K) | solid | 16.4 J/(mol K) specific free energy of formation Δ_fG° | gas | 31.8 kJ/g molar free energy of formation Δ_fG° | gas | 286.6 kJ/mol specific heat of formation Δ_fH° | gas | 35.95 kJ/g | solid | 0 kJ/g molar heat of formation Δ_fH° | gas | 324 kJ/mol | solid | 0 kJ/mol specific entropy S° | solid | 1.11 J/(g K) molar entropy S° | solid | 10 J/(mol K) molar heat of vaporization | 297 kJ/mol | specific heat of vaporization | 33 kJ/g | molar heat of fusion | 7.895 kJ/mol | specific heat of fusion | 0.876 kJ/g | critical temperature | 5205 K | (at STP)
Chemical identifiers
![CAS number | 7440-41-7 PubChem CID number | 5460467 PubChem SID number | 24856053 SMILES identifier | [Be] InChI identifier | InChI=1/Be RTECS number | DS1750000 MDL number | MFCD00134032](../image_source/1f9d5b05cdaa86f23e61db14e81ea9c2.png)
CAS number | 7440-41-7 PubChem CID number | 5460467 PubChem SID number | 24856053 SMILES identifier | [Be] InChI identifier | InChI=1/Be RTECS number | DS1750000 MDL number | MFCD00134032
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 1 NFPA reactivity rating | 0
Toxicity properties

long-term exposure limit | 0.002 mg/m^3 (over 8 hours) RTECS classes | tumorigen | mutagen
Units
