Input interpretation

synthetic elements | molar mass
Summary
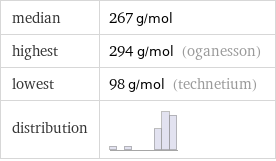
median | 267 g/mol highest | 294 g/mol (oganesson) lowest | 98 g/mol (technetium) distribution |
Units

Distribution plots
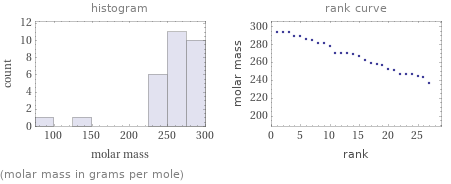
(molar mass in grams per mole)
Molar mass rankings
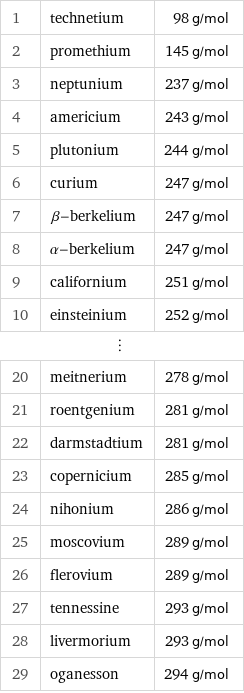
1 | technetium | 98 g/mol 2 | promethium | 145 g/mol 3 | neptunium | 237 g/mol 4 | americium | 243 g/mol 5 | plutonium | 244 g/mol 6 | curium | 247 g/mol 7 | β-berkelium | 247 g/mol 8 | α-berkelium | 247 g/mol 9 | californium | 251 g/mol 10 | einsteinium | 252 g/mol ⋮ | | 20 | meitnerium | 278 g/mol 21 | roentgenium | 281 g/mol 22 | darmstadtium | 281 g/mol 23 | copernicium | 285 g/mol 24 | nihonium | 286 g/mol 25 | moscovium | 289 g/mol 26 | flerovium | 289 g/mol 27 | tennessine | 293 g/mol 28 | livermorium | 293 g/mol 29 | oganesson | 294 g/mol
Unit conversion for median molar mass 267 g/mol
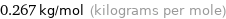
0.267 kg/mol (kilograms per mole)
Comparisons for median molar mass 267 g/mol

≈ 0.37 × molar mass of fullerene ( ≈ 721 g/mol )
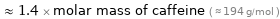
≈ 1.4 × molar mass of caffeine ( ≈ 194 g/mol )
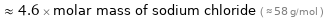
≈ 4.6 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
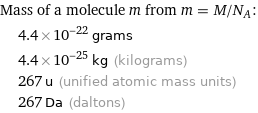
Mass of a molecule m from m = M/N_A: | 4.4×10^-22 grams | 4.4×10^-25 kg (kilograms) | 267 u (unified atomic mass units) | 267 Da (daltons)
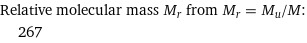
Relative molecular mass M_r from M_r = M_u/M: | 267