Input interpretation
diamond (mineral) | barringerite (mineral)
Images

Images
General properties
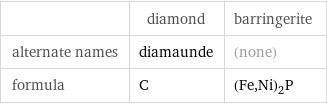
| diamond | barringerite alternate names | diamaunde | (none) formula | C | (Fe, Ni)_2P
Basic properties
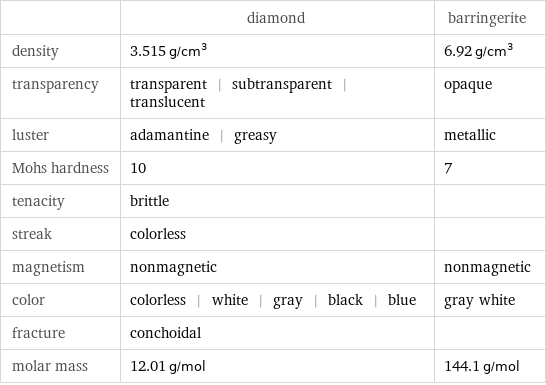
| diamond | barringerite density | 3.515 g/cm^3 | 6.92 g/cm^3 transparency | transparent | subtransparent | translucent | opaque luster | adamantine | greasy | metallic Mohs hardness | 10 | 7 tenacity | brittle | streak | colorless | magnetism | nonmagnetic | nonmagnetic color | colorless | white | gray | black | blue | gray white fracture | conchoidal | molar mass | 12.01 g/mol | 144.1 g/mol
Units

Mineral identifiers
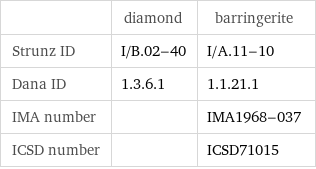
| diamond | barringerite Strunz ID | I/B.02-40 | I/A.11-10 Dana ID | 1.3.6.1 | 1.1.21.1 IMA number | | IMA1968-037 ICSD number | | ICSD71015
Crystallographic properties
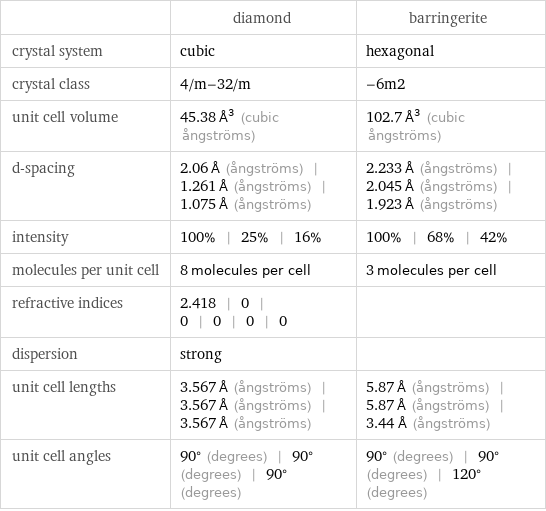
| diamond | barringerite crystal system | cubic | hexagonal crystal class | 4/m-32/m | -6m2 unit cell volume | 45.38 Å^3 (cubic ångströms) | 102.7 Å^3 (cubic ångströms) d-spacing | 2.06 Å (ångströms) | 1.261 Å (ångströms) | 1.075 Å (ångströms) | 2.233 Å (ångströms) | 2.045 Å (ångströms) | 1.923 Å (ångströms) intensity | 100% | 25% | 16% | 100% | 68% | 42% molecules per unit cell | 8 molecules per cell | 3 molecules per cell refractive indices | 2.418 | 0 | 0 | 0 | 0 | 0 | dispersion | strong | unit cell lengths | 3.567 Å (ångströms) | 3.567 Å (ångströms) | 3.567 Å (ångströms) | 5.87 Å (ångströms) | 5.87 Å (ångströms) | 3.44 Å (ångströms) unit cell angles | 90° (degrees) | 90° (degrees) | 90° (degrees) | 90° (degrees) | 90° (degrees) | 120° (degrees)
Wikipedia summary
Diamond

Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. At room temperature and pressure, another solid form of carbon known as graphite is the chemically stable form, but diamond almost never converts to it. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are utilized in major industrial applications such as cutting and polishing tools. They are also the reason that diamond anvil cells can subject materials to pressures found deep in the Earth.