Input interpretation

dying low mass star elements | molar mass
Summary
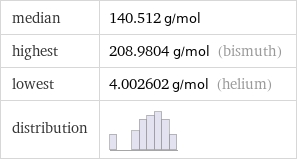
median | 140.512 g/mol highest | 208.9804 g/mol (bismuth) lowest | 4.002602 g/mol (helium) distribution |
Units

Distribution plots
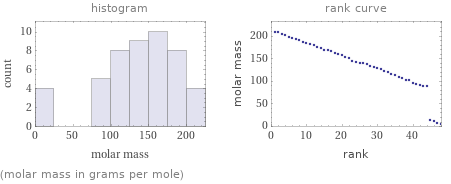
(molar mass in grams per mole)
Molar mass rankings
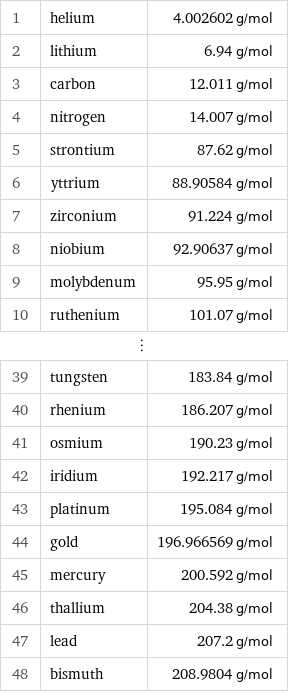
1 | helium | 4.002602 g/mol 2 | lithium | 6.94 g/mol 3 | carbon | 12.011 g/mol 4 | nitrogen | 14.007 g/mol 5 | strontium | 87.62 g/mol 6 | yttrium | 88.90584 g/mol 7 | zirconium | 91.224 g/mol 8 | niobium | 92.90637 g/mol 9 | molybdenum | 95.95 g/mol 10 | ruthenium | 101.07 g/mol ⋮ | | 39 | tungsten | 183.84 g/mol 40 | rhenium | 186.207 g/mol 41 | osmium | 190.23 g/mol 42 | iridium | 192.217 g/mol 43 | platinum | 195.084 g/mol 44 | gold | 196.966569 g/mol 45 | mercury | 200.592 g/mol 46 | thallium | 204.38 g/mol 47 | lead | 207.2 g/mol 48 | bismuth | 208.9804 g/mol
Unit conversion for median molar mass 140.512 g/mol
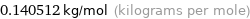
0.140512 kg/mol (kilograms per mole)
Comparisons for median molar mass 140.512 g/mol
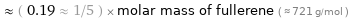
≈ ( 0.19 ≈ 1/5 ) × molar mass of fullerene ( ≈ 721 g/mol )
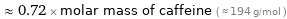
≈ 0.72 × molar mass of caffeine ( ≈ 194 g/mol )
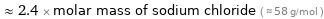
≈ 2.4 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
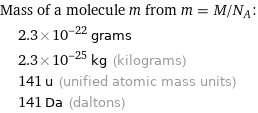
Mass of a molecule m from m = M/N_A: | 2.3×10^-22 grams | 2.3×10^-25 kg (kilograms) | 141 u (unified atomic mass units) | 141 Da (daltons)
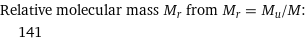
Relative molecular mass M_r from M_r = M_u/M: | 141