Input interpretation

f-block elements | in elements | molar mass
Summary
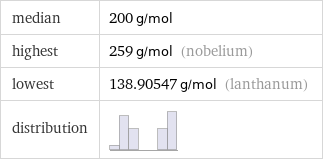
median | 200 g/mol highest | 259 g/mol (nobelium) lowest | 138.90547 g/mol (lanthanum) distribution |
Units

Distribution plots
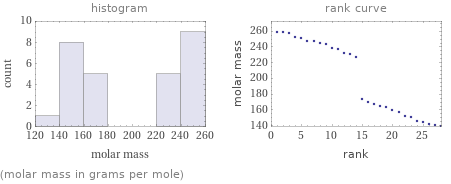
(molar mass in grams per mole)
Molar mass rankings
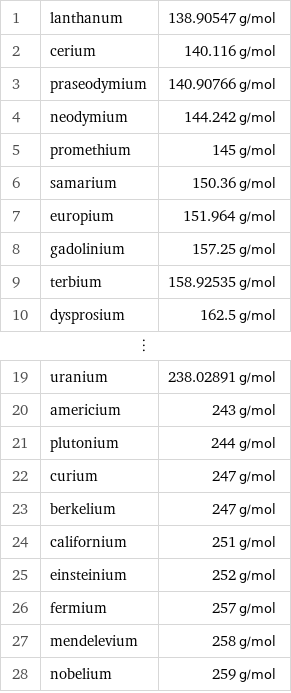
1 | lanthanum | 138.90547 g/mol 2 | cerium | 140.116 g/mol 3 | praseodymium | 140.90766 g/mol 4 | neodymium | 144.242 g/mol 5 | promethium | 145 g/mol 6 | samarium | 150.36 g/mol 7 | europium | 151.964 g/mol 8 | gadolinium | 157.25 g/mol 9 | terbium | 158.92535 g/mol 10 | dysprosium | 162.5 g/mol ⋮ | | 19 | uranium | 238.02891 g/mol 20 | americium | 243 g/mol 21 | plutonium | 244 g/mol 22 | curium | 247 g/mol 23 | berkelium | 247 g/mol 24 | californium | 251 g/mol 25 | einsteinium | 252 g/mol 26 | fermium | 257 g/mol 27 | mendelevium | 258 g/mol 28 | nobelium | 259 g/mol
Unit conversion
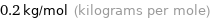
0.2 kg/mol (kilograms per mole)
Comparisons
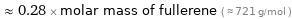
≈ 0.28 × molar mass of fullerene ( ≈ 721 g/mol )
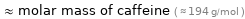
≈ molar mass of caffeine ( ≈ 194 g/mol )
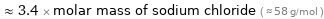
≈ 3.4 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
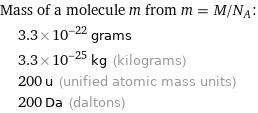
Mass of a molecule m from m = M/N_A: | 3.3×10^-22 grams | 3.3×10^-25 kg (kilograms) | 200 u (unified atomic mass units) | 200 Da (daltons)
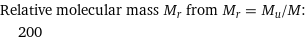
Relative molecular mass M_r from M_r = M_u/M: | 200