Input interpretation

polar solvents | vapor pressure
Summary
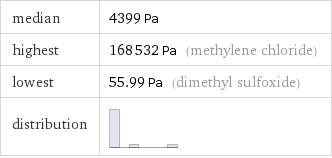
median | 4399 Pa highest | 168532 Pa (methylene chloride) lowest | 55.99 Pa (dimethyl sulfoxide) distribution |
Units

Distribution plots
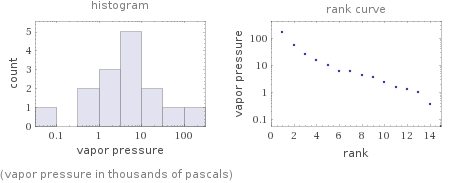
(vapor pressure in thousands of pascals)
Vapor pressure rankings

1 | dimethyl sulfoxide | 55.99 Pa 2 | N, N-dimethylformamide | 359.9 Pa 3 | 1-butanol | 965 Pa 4 | N-propanol | 1333 Pa 5 | acetic acid | 1520 Pa 6 | water | 2339 Pa 7 | 1, 4-dioxane | 3599 Pa 8 | isopropanol | 4399 Pa 9 | ethanol | 5945 Pa 10 | formic acid | 5972 Pa 11 | acetonitrile | 9704 Pa 12 | tetrahydrofuran | 15196 Pa 13 | acetone | 24527 Pa 14 | methanol | 54653 Pa 15 | methylene chloride | 168532 Pa
Unit conversions for median vapor pressure 4399 Pa
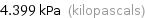
4.399 kPa (kilopascals)
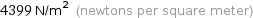
4399 N/m^2 (newtons per square meter)

448.6 kgf/m^2 (kilograms-force per square meter)
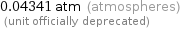
0.04341 atm (atmospheres) (unit officially deprecated)

0.04486 at (technical atmospheres) (unit officially deprecated)
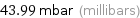
43.99 mbar (millibars)
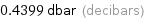
0.4399 dbar (decibars)
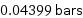
0.04399 bars
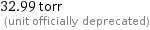
32.99 torr (unit officially deprecated)
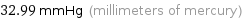
32.99 mmHg (millimeters of mercury)
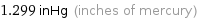
1.299 inHg (inches of mercury)

44.86 cm WC (centimeters of water column)

448.6 mm WC (millimeters of water column)

0.4486 m WC (meters of water column)
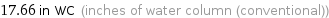
17.66 in WC (inches of water column (conventional))
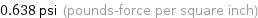
0.638 psi (pounds-force per square inch)

91.87 lbf/ft^2 (pounds-force per square foot)
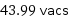
43.99 vacs
Comparisons for median vapor pressure 4399 Pa
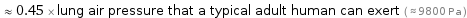
≈ 0.45 × lung air pressure that a typical adult human can exert ( ≈ 9800 Pa )
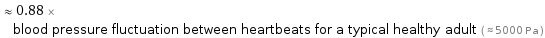
≈ 0.88 × blood pressure fluctuation between heartbeats for a typical healthy adult ( ≈ 5000 Pa )
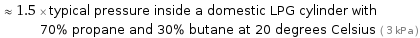
≈ 1.5 × typical pressure inside a domestic LPG cylinder with 70% propane and 30% butane at 20 degrees Celsius ( 3 kPa )
Corresponding quantities

Depth of water d giving gauge pressure p_e from p_e = ρ_(H_2O)gh: | 45 cm (centimeters) | 1.5 feet | 18 inches | (assuming maximum water density ≈ 1000 kg/m^3)

Standard Atmosphere altitude: | 21 km (kilometers) | 13 miles | (based on 1976 US Standard Atmosphere)