Input interpretation
zinc fluoride
Chemical names and formulas
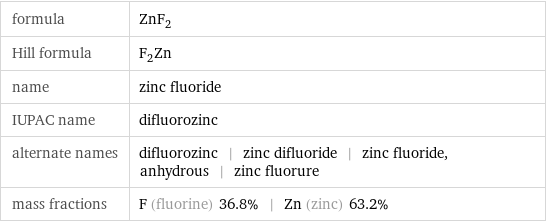
formula | ZnF_2 Hill formula | F_2Zn name | zinc fluoride IUPAC name | difluorozinc alternate names | difluorozinc | zinc difluoride | zinc fluoride, anhydrous | zinc fluorure mass fractions | F (fluorine) 36.8% | Zn (zinc) 63.2%
Structure diagram

Structure diagram
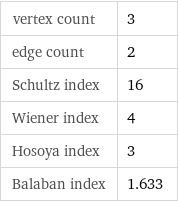
vertex count | 3 edge count | 2 Schultz index | 16 Wiener index | 4 Hosoya index | 3 Balaban index | 1.633
Basic properties
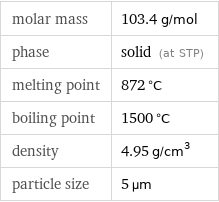
molar mass | 103.4 g/mol phase | solid (at STP) melting point | 872 °C boiling point | 1500 °C density | 4.95 g/cm^3 particle size | 5 µm
Solid properties (at STP)
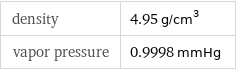
density | 4.95 g/cm^3 vapor pressure | 0.9998 mmHg
Units

Thermodynamic properties
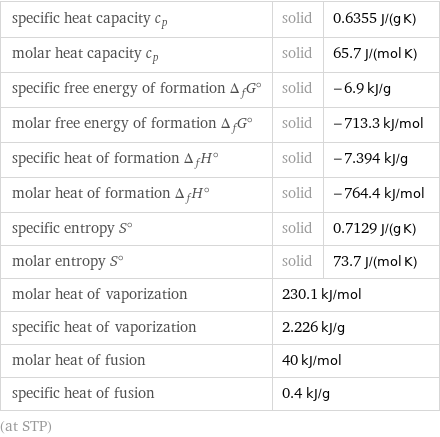
specific heat capacity c_p | solid | 0.6355 J/(g K) molar heat capacity c_p | solid | 65.7 J/(mol K) specific free energy of formation Δ_fG° | solid | -6.9 kJ/g molar free energy of formation Δ_fG° | solid | -713.3 kJ/mol specific heat of formation Δ_fH° | solid | -7.394 kJ/g molar heat of formation Δ_fH° | solid | -764.4 kJ/mol specific entropy S° | solid | 0.7129 J/(g K) molar entropy S° | solid | 73.7 J/(mol K) molar heat of vaporization | 230.1 kJ/mol | specific heat of vaporization | 2.226 kJ/g | molar heat of fusion | 40 kJ/mol | specific heat of fusion | 0.4 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7783-49-5 PubChem CID number | 24551 PubChem SID number | 24852207 SMILES identifier | F[Zn]F InChI identifier | InChI=1/2FH.Zn/h2*1H;/q;;+2/p-2/f2F.Zn/h2*1h;/q2*-1;m RTECS number | ZH3500000 MDL number | MFCD00011298](../image_source/bac3ac9990ff6ccf53cd89f2dfd65f27.png)
CAS number | 7783-49-5 PubChem CID number | 24551 PubChem SID number | 24852207 SMILES identifier | F[Zn]F InChI identifier | InChI=1/2FH.Zn/h2*1H;/q;;+2/p-2/f2F.Zn/h2*1h;/q2*-1;m RTECS number | ZH3500000 MDL number | MFCD00011298
Toxicity properties

RTECS classes | other