Input interpretation

Lewis acids | vapor pressure
Summary
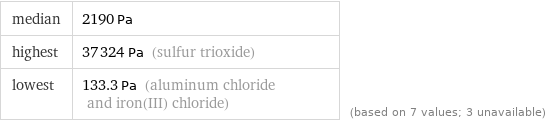
median | 2190 Pa highest | 37324 Pa (sulfur trioxide) lowest | 133.3 Pa (aluminum chloride and iron(III) chloride) | (based on 7 values; 3 unavailable)
Entities with missing values
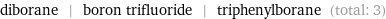
diborane | boron trifluoride | triphenylborane (total: 3)
Vapor pressure rankings
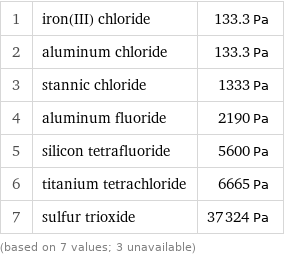
1 | iron(III) chloride | 133.3 Pa 2 | aluminum chloride | 133.3 Pa 3 | stannic chloride | 1333 Pa 4 | aluminum fluoride | 2190 Pa 5 | silicon tetrafluoride | 5600 Pa 6 | titanium tetrachloride | 6665 Pa 7 | sulfur trioxide | 37324 Pa (based on 7 values; 3 unavailable)
Unit conversions for median vapor pressure 2190 Pa
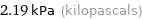
2.19 kPa (kilopascals)

2190 N/m^2 (newtons per square meter)
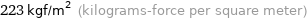
223 kgf/m^2 (kilograms-force per square meter)
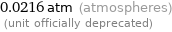
0.0216 atm (atmospheres) (unit officially deprecated)

0.0223 at (technical atmospheres) (unit officially deprecated)
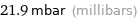
21.9 mbar (millibars)
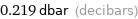
0.219 dbar (decibars)
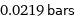
0.0219 bars
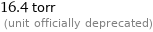
16.4 torr (unit officially deprecated)
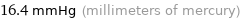
16.4 mmHg (millimeters of mercury)
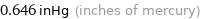
0.646 inHg (inches of mercury)

22.3 cm WC (centimeters of water column)

223 mm WC (millimeters of water column)
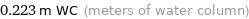
0.223 m WC (meters of water column)

8.78 in WC (inches of water column (conventional))
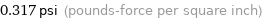
0.317 psi (pounds-force per square inch)
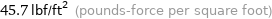
45.7 lbf/ft^2 (pounds-force per square foot)
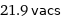
21.9 vacs
Comparisons for median vapor pressure 2190 Pa
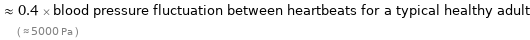
≈ 0.4 × blood pressure fluctuation between heartbeats for a typical healthy adult ( ≈ 5000 Pa )

≈ 0.73 × typical pressure inside a domestic LPG cylinder with 70% propane and 30% butane at 20 degrees Celsius ( 3 kPa )

≈ (0.2 to 2.2) × approximate explosion peak overpressure needed to break a glass window ( 1000 to 10000 Pa )
Corresponding quantities
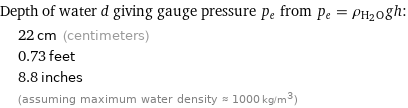
Depth of water d giving gauge pressure p_e from p_e = ρ_(H_2O)gh: | 22 cm (centimeters) | 0.73 feet | 8.8 inches | (assuming maximum water density ≈ 1000 kg/m^3)

Standard Atmosphere altitude: | 26 km (kilometers) | 16 miles | (based on 1976 US Standard Atmosphere)