Input interpretation
barium hydride | nickel(II) sulfamate tetrahydrate
Chemical names and formulas
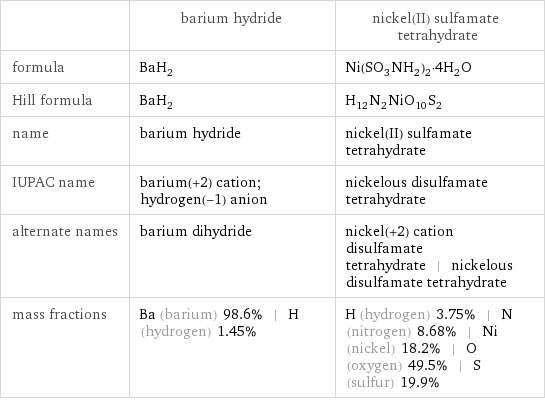
| barium hydride | nickel(II) sulfamate tetrahydrate formula | BaH_2 | Ni(SO_3NH_2)_2·4H_2O Hill formula | BaH_2 | H_12N_2NiO_10S_2 name | barium hydride | nickel(II) sulfamate tetrahydrate IUPAC name | barium(+2) cation; hydrogen(-1) anion | nickelous disulfamate tetrahydrate alternate names | barium dihydride | nickel(+2) cation disulfamate tetrahydrate | nickelous disulfamate tetrahydrate mass fractions | Ba (barium) 98.6% | H (hydrogen) 1.45% | H (hydrogen) 3.75% | N (nitrogen) 8.68% | Ni (nickel) 18.2% | O (oxygen) 49.5% | S (sulfur) 19.9%
Structure diagrams
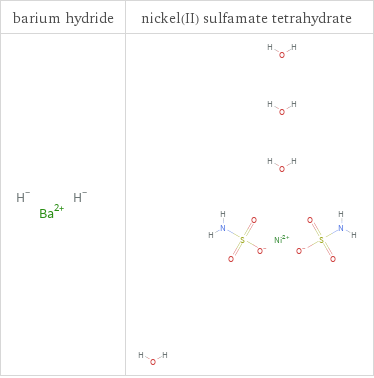
Structure diagrams
Basic properties

| barium hydride | nickel(II) sulfamate tetrahydrate molar mass | 139.343 g/mol | 322.91 g/mol phase | solid (at STP) | melting point | 1200 °C | density | 4.21 g/cm^3 |
Units
