Input interpretation
methyl chloride | 1, 1, 1-trichloroethane
Chemical names and formulas
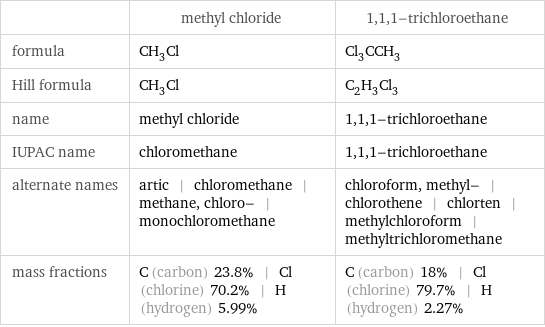
| methyl chloride | 1, 1, 1-trichloroethane formula | CH_3Cl | Cl_3CCH_3 Hill formula | CH_3Cl | C_2H_3Cl_3 name | methyl chloride | 1, 1, 1-trichloroethane IUPAC name | chloromethane | 1, 1, 1-trichloroethane alternate names | artic | chloromethane | methane, chloro- | monochloromethane | chloroform, methyl- | chlorothene | chlorten | methylchloroform | methyltrichloromethane mass fractions | C (carbon) 23.8% | Cl (chlorine) 70.2% | H (hydrogen) 5.99% | C (carbon) 18% | Cl (chlorine) 79.7% | H (hydrogen) 2.27%
Structure diagrams
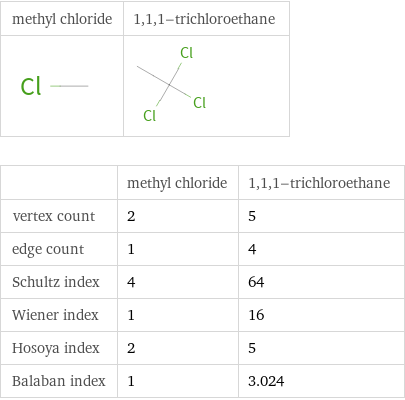
| methyl chloride | 1, 1, 1-trichloroethane vertex count | 2 | 5 edge count | 1 | 4 Schultz index | 4 | 64 Wiener index | 1 | 16 Hosoya index | 2 | 5 Balaban index | 1 | 3.024
3D structure
3D structure
Basic properties
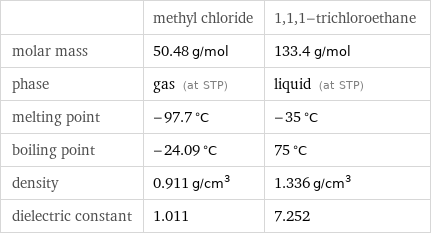
| methyl chloride | 1, 1, 1-trichloroethane molar mass | 50.48 g/mol | 133.4 g/mol phase | gas (at STP) | liquid (at STP) melting point | -97.7 °C | -35 °C boiling point | -24.09 °C | 75 °C density | 0.911 g/cm^3 | 1.336 g/cm^3 dielectric constant | 1.011 | 7.252
Gas properties
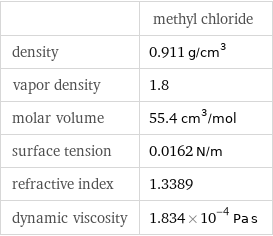
| methyl chloride density | 0.911 g/cm^3 vapor density | 1.8 molar volume | 55.4 cm^3/mol surface tension | 0.0162 N/m refractive index | 1.3389 dynamic viscosity | 1.834×10^-4 Pa s
Units

Liquid properties
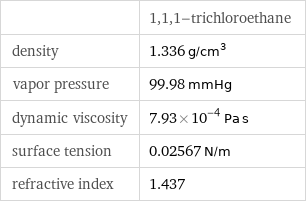
| 1, 1, 1-trichloroethane density | 1.336 g/cm^3 vapor pressure | 99.98 mmHg dynamic viscosity | 7.93×10^-4 Pa s surface tension | 0.02567 N/m refractive index | 1.437
Units
Thermodynamic properties
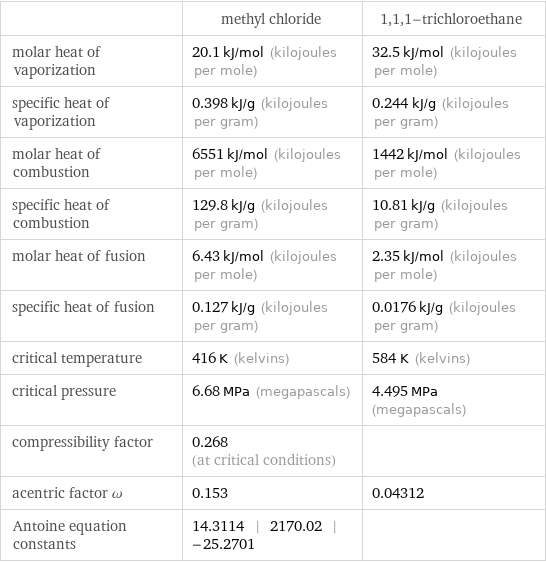
| methyl chloride | 1, 1, 1-trichloroethane molar heat of vaporization | 20.1 kJ/mol (kilojoules per mole) | 32.5 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.398 kJ/g (kilojoules per gram) | 0.244 kJ/g (kilojoules per gram) molar heat of combustion | 6551 kJ/mol (kilojoules per mole) | 1442 kJ/mol (kilojoules per mole) specific heat of combustion | 129.8 kJ/g (kilojoules per gram) | 10.81 kJ/g (kilojoules per gram) molar heat of fusion | 6.43 kJ/mol (kilojoules per mole) | 2.35 kJ/mol (kilojoules per mole) specific heat of fusion | 0.127 kJ/g (kilojoules per gram) | 0.0176 kJ/g (kilojoules per gram) critical temperature | 416 K (kelvins) | 584 K (kelvins) critical pressure | 6.68 MPa (megapascals) | 4.495 MPa (megapascals) compressibility factor | 0.268 (at critical conditions) | acentric factor ω | 0.153 | 0.04312 Antoine equation constants | 14.3114 | 2170.02 | -25.2701 |
Chemical identifiers
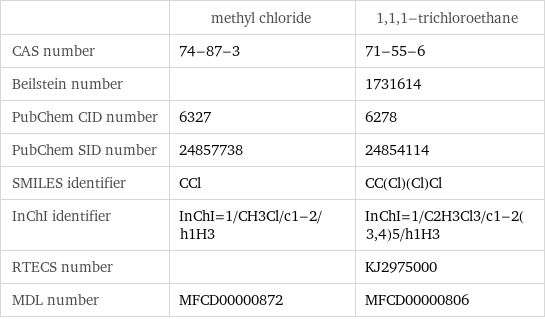
| methyl chloride | 1, 1, 1-trichloroethane CAS number | 74-87-3 | 71-55-6 Beilstein number | | 1731614 PubChem CID number | 6327 | 6278 PubChem SID number | 24857738 | 24854114 SMILES identifier | CCl | CC(Cl)(Cl)Cl InChI identifier | InChI=1/CH3Cl/c1-2/h1H3 | InChI=1/C2H3Cl3/c1-2(3, 4)5/h1H3 RTECS number | | KJ2975000 MDL number | MFCD00000872 | MFCD00000806
NFPA label
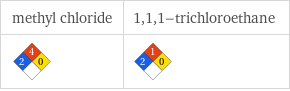
NFPA label
Safety properties
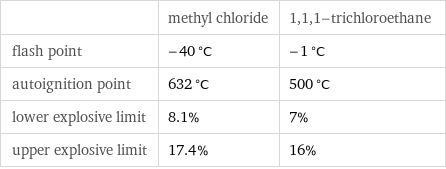
| methyl chloride | 1, 1, 1-trichloroethane flash point | -40 °C | -1 °C autoignition point | 632 °C | 500 °C lower explosive limit | 8.1% | 7% upper explosive limit | 17.4% | 16%