Input interpretation
cadmium cyanide | ammonium trifluoroacetate
Chemical names and formulas
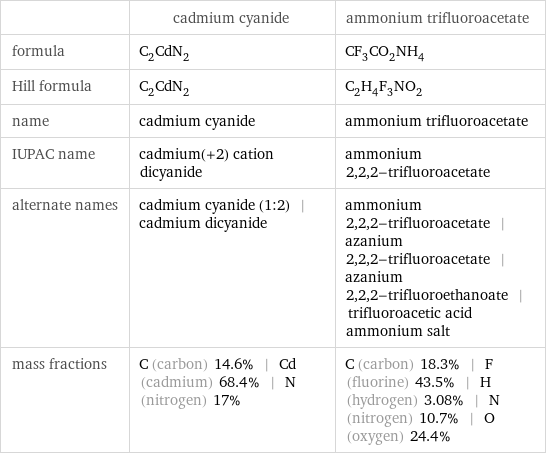
| cadmium cyanide | ammonium trifluoroacetate formula | C_2CdN_2 | CF_3CO_2NH_4 Hill formula | C_2CdN_2 | C_2H_4F_3NO_2 name | cadmium cyanide | ammonium trifluoroacetate IUPAC name | cadmium(+2) cation dicyanide | ammonium 2, 2, 2-trifluoroacetate alternate names | cadmium cyanide (1:2) | cadmium dicyanide | ammonium 2, 2, 2-trifluoroacetate | azanium 2, 2, 2-trifluoroacetate | azanium 2, 2, 2-trifluoroethanoate | trifluoroacetic acid ammonium salt mass fractions | C (carbon) 14.6% | Cd (cadmium) 68.4% | N (nitrogen) 17% | C (carbon) 18.3% | F (fluorine) 43.5% | H (hydrogen) 3.08% | N (nitrogen) 10.7% | O (oxygen) 24.4%
Structure diagrams
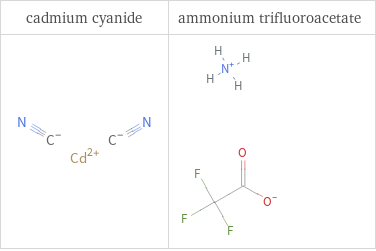
Structure diagrams
Basic properties
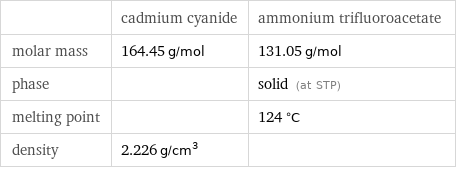
| cadmium cyanide | ammonium trifluoroacetate molar mass | 164.45 g/mol | 131.05 g/mol phase | | solid (at STP) melting point | | 124 °C density | 2.226 g/cm^3 |
Units
