Input interpretation

period 2 elements | phase at STP
Summary
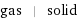
gas | solid
Categorized list
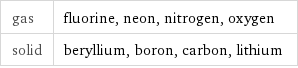
gas | fluorine, neon, nitrogen, oxygen solid | beryllium, boron, carbon, lithium
Thermodynamic properties
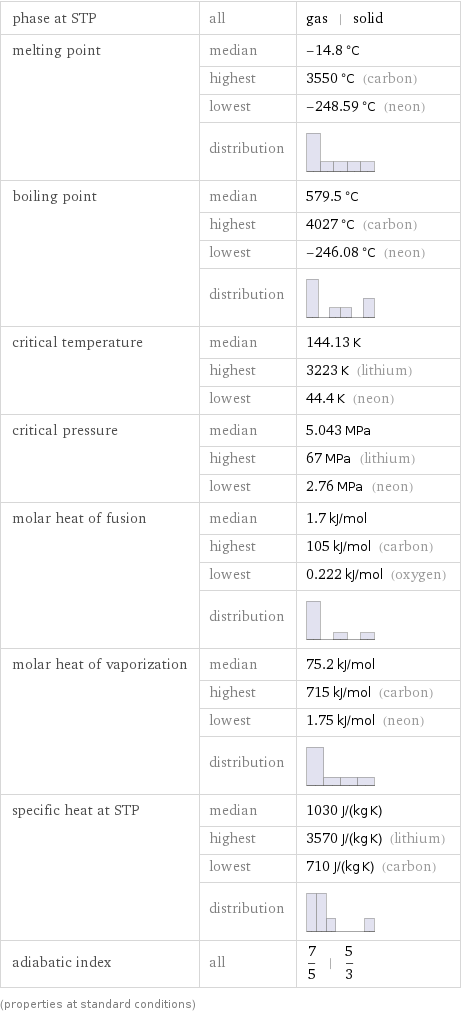
phase at STP | all | gas | solid melting point | median | -14.8 °C | highest | 3550 °C (carbon) | lowest | -248.59 °C (neon) | distribution | boiling point | median | 579.5 °C | highest | 4027 °C (carbon) | lowest | -246.08 °C (neon) | distribution | critical temperature | median | 144.13 K | highest | 3223 K (lithium) | lowest | 44.4 K (neon) critical pressure | median | 5.043 MPa | highest | 67 MPa (lithium) | lowest | 2.76 MPa (neon) molar heat of fusion | median | 1.7 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) | distribution | molar heat of vaporization | median | 75.2 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 1.75 kJ/mol (neon) | distribution | specific heat at STP | median | 1030 J/(kg K) | highest | 3570 J/(kg K) (lithium) | lowest | 710 J/(kg K) (carbon) | distribution | adiabatic index | all | 7/5 | 5/3 (properties at standard conditions)