Input interpretation

halogenated compounds | molar heat of vaporization
Summary
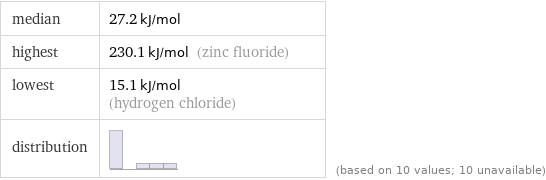
median | 27.2 kJ/mol highest | 230.1 kJ/mol (zinc fluoride) lowest | 15.1 kJ/mol (hydrogen chloride) distribution | | (based on 10 values; 10 unavailable)
Entities with missing values
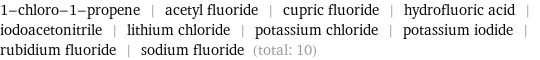
1-chloro-1-propene | acetyl fluoride | cupric fluoride | hydrofluoric acid | iodoacetonitrile | lithium chloride | potassium chloride | potassium iodide | rubidium fluoride | sodium fluoride (total: 10)
Distribution plots
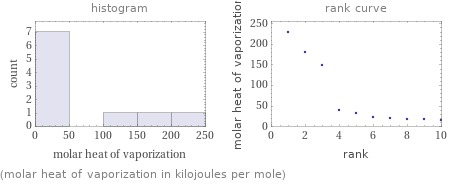
(molar heat of vaporization in kilojoules per mole)
Molar heat of vaporization rankings
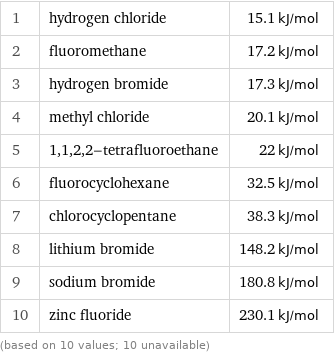
1 | hydrogen chloride | 15.1 kJ/mol 2 | fluoromethane | 17.2 kJ/mol 3 | hydrogen bromide | 17.3 kJ/mol 4 | methyl chloride | 20.1 kJ/mol 5 | 1, 1, 2, 2-tetrafluoroethane | 22 kJ/mol 6 | fluorocyclohexane | 32.5 kJ/mol 7 | chlorocyclopentane | 38.3 kJ/mol 8 | lithium bromide | 148.2 kJ/mol 9 | sodium bromide | 180.8 kJ/mol 10 | zinc fluoride | 230.1 kJ/mol (based on 10 values; 10 unavailable)
Unit conversions for median molar heat of vaporization 27.2 kJ/mol
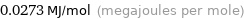
0.0273 MJ/mol (megajoules per mole)
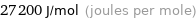
27200 J/mol (joules per mole)

6.51 kcal/mol (thermochemical kilocalories per mole)
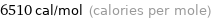
6510 cal/mol (calories per mole)

0.282 eV (molar electronvolts)