Input interpretation

carbides | molar mass
Summary
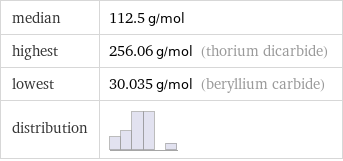
median | 112.5 g/mol highest | 256.06 g/mol (thorium dicarbide) lowest | 30.035 g/mol (beryllium carbide) distribution |
Units

Distribution plots
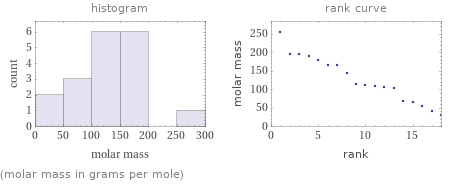
(molar mass in grams per mole)
Molar mass rankings
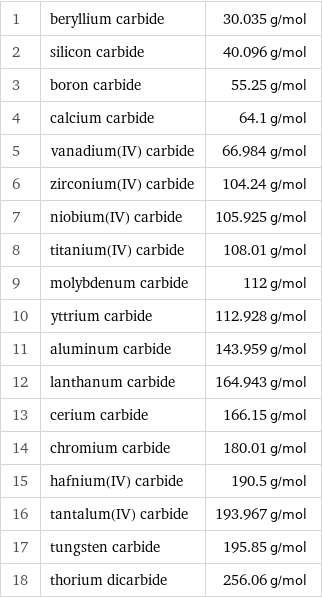
1 | beryllium carbide | 30.035 g/mol 2 | silicon carbide | 40.096 g/mol 3 | boron carbide | 55.25 g/mol 4 | calcium carbide | 64.1 g/mol 5 | vanadium(IV) carbide | 66.984 g/mol 6 | zirconium(IV) carbide | 104.24 g/mol 7 | niobium(IV) carbide | 105.925 g/mol 8 | titanium(IV) carbide | 108.01 g/mol 9 | molybdenum carbide | 112 g/mol 10 | yttrium carbide | 112.928 g/mol 11 | aluminum carbide | 143.959 g/mol 12 | lanthanum carbide | 164.943 g/mol 13 | cerium carbide | 166.15 g/mol 14 | chromium carbide | 180.01 g/mol 15 | hafnium(IV) carbide | 190.5 g/mol 16 | tantalum(IV) carbide | 193.967 g/mol 17 | tungsten carbide | 195.85 g/mol 18 | thorium dicarbide | 256.06 g/mol
Unit conversion for median molar mass 112.5 g/mol
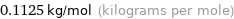
0.1125 kg/mol (kilograms per mole)
Comparisons for median molar mass 112.5 g/mol
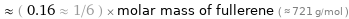
≈ ( 0.16 ≈ 1/6 ) × molar mass of fullerene ( ≈ 721 g/mol )
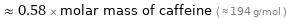
≈ 0.58 × molar mass of caffeine ( ≈ 194 g/mol )
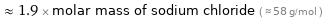
≈ 1.9 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
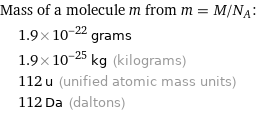
Mass of a molecule m from m = M/N_A: | 1.9×10^-22 grams | 1.9×10^-25 kg (kilograms) | 112 u (unified atomic mass units) | 112 Da (daltons)
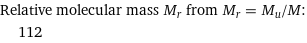
Relative molecular mass M_r from M_r = M_u/M: | 112