Input interpretation

vanadium | melting point
Result
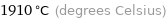
1910 °C (degrees Celsius)
Unit conversions
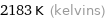
2183 K (kelvins)

3470 °F (degrees Fahrenheit)
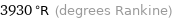
3930 °R (degrees Rankine)

1528 °Ré (degrees Réaumur)
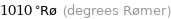
1010 °Rø (degrees Rømer)
Comparisons as temperature
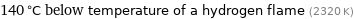
140 °C below temperature of a hydrogen flame (2320 K)
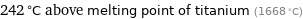
242 °C above melting point of titanium (1668 °C)
Blackbody information
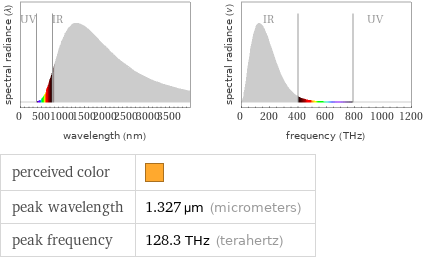
perceived color | peak wavelength | 1.327 µm (micrometers) peak frequency | 128.3 THz (terahertz)
Corresponding quantities
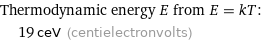
Thermodynamic energy E from E = kT: | 19 ceV (centielectronvolts)
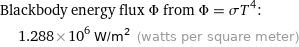
Blackbody energy flux Φ from Φ = σT^4: | 1.288×10^6 W/m^2 (watts per square meter)

Approximate luminous exitance from a planar blackbody radiator perpendicular to its surface: | 4.135×10^6 lx (lux)
Thermodynamic properties
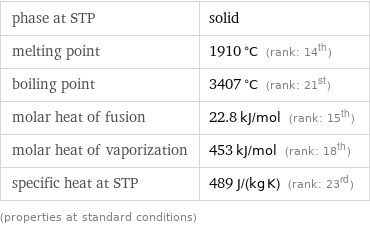
phase at STP | solid melting point | 1910 °C (rank: 14th) boiling point | 3407 °C (rank: 21st) molar heat of fusion | 22.8 kJ/mol (rank: 15th) molar heat of vaporization | 453 kJ/mol (rank: 18th) specific heat at STP | 489 J/(kg K) (rank: 23rd) (properties at standard conditions)