Input interpretation
![2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde](../image_source/fc6647a465aa721fb55e17246207d17f.png)
2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde
Basic properties
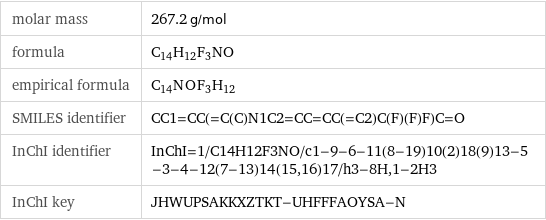
molar mass | 267.2 g/mol formula | C_14H_12F_3NO empirical formula | C_14N_O_F_3H_12 SMILES identifier | CC1=CC(=C(C)N1C2=CC=CC(=C2)C(F)(F)F)C=O InChI identifier | InChI=1/C14H12F3NO/c1-9-6-11(8-19)10(2)18(9)13-5-3-4-12(7-13)14(15, 16)17/h3-8H, 1-2H3 InChI key | JHWUPSAKKXZTKT-UHFFFAOYSA-N
Lewis structure
![Draw the Lewis structure of 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), fluorine (n_F, val = 7), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 14 n_C, val + 3 n_F, val + 12 n_H, val + n_N, val + n_O, val = 100 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), fluorine (n_F, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 14 n_C, full + 3 n_F, full + 12 n_H, full + n_N, full + n_O, full = 176 Subtracting these two numbers shows that 176 - 100 = 76 bonding electrons are needed. Each bond has two electrons, so in addition to the 32 bonds already present in the diagram add 6 bonds. To minimize formal charge carbon wants 4 bonds and oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 6 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |](../image_source/683ee07489273737f418b7b6905b9c13.png)
Draw the Lewis structure of 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), fluorine (n_F, val = 7), hydrogen (n_H, val = 1), nitrogen (n_N, val = 5), and oxygen (n_O, val = 6) atoms: 14 n_C, val + 3 n_F, val + 12 n_H, val + n_N, val + n_O, val = 100 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), fluorine (n_F, full = 8), hydrogen (n_H, full = 2), nitrogen (n_N, full = 8), and oxygen (n_O, full = 8): 14 n_C, full + 3 n_F, full + 12 n_H, full + n_N, full + n_O, full = 176 Subtracting these two numbers shows that 176 - 100 = 76 bonding electrons are needed. Each bond has two electrons, so in addition to the 32 bonds already present in the diagram add 6 bonds. To minimize formal charge carbon wants 4 bonds and oxygen wants 2 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 6 bonds by pairing electrons between adjacent highlighted atoms. Note that the six atom ring is aromatic, so that the single and double bonds may be rearranged: Answer: | |
Estimated thermodynamic properties
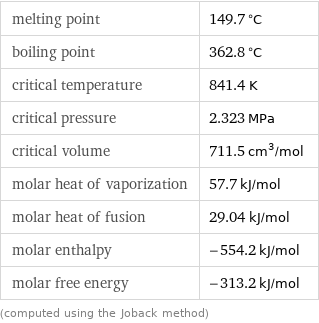
melting point | 149.7 °C boiling point | 362.8 °C critical temperature | 841.4 K critical pressure | 2.323 MPa critical volume | 711.5 cm^3/mol molar heat of vaporization | 57.7 kJ/mol molar heat of fusion | 29.04 kJ/mol molar enthalpy | -554.2 kJ/mol molar free energy | -313.2 kJ/mol (computed using the Joback method)
Units

Quantitative molecular descriptors
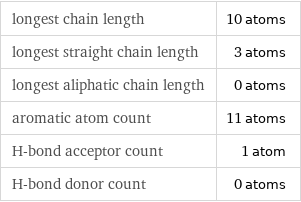
longest chain length | 10 atoms longest straight chain length | 3 atoms longest aliphatic chain length | 0 atoms aromatic atom count | 11 atoms H-bond acceptor count | 1 atom H-bond donor count | 0 atoms
Elemental composition
![Find the elemental composition for 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_14H_12F_3NO Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms, per molecule: | number of atoms C (carbon) | 14 N (nitrogen) | 1 O (oxygen) | 1 F (fluorine) | 3 H (hydrogen) | 12 N_atoms = 14 + 1 + 1 + 3 + 12 = 31 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction C (carbon) | 14 | 14/31 N (nitrogen) | 1 | 1/31 O (oxygen) | 1 | 1/31 F (fluorine) | 3 | 3/31 H (hydrogen) | 12 | 12/31 Check: 14/31 + 1/31 + 1/31 + 3/31 + 12/31 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent C (carbon) | 14 | 14/31 × 100% = 45.2% N (nitrogen) | 1 | 1/31 × 100% = 3.23% O (oxygen) | 1 | 1/31 × 100% = 3.23% F (fluorine) | 3 | 3/31 × 100% = 9.68% H (hydrogen) | 12 | 12/31 × 100% = 38.7% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u C (carbon) | 14 | 45.2% | 12.011 N (nitrogen) | 1 | 3.23% | 14.007 O (oxygen) | 1 | 3.23% | 15.999 F (fluorine) | 3 | 9.68% | 18.998403163 H (hydrogen) | 12 | 38.7% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u C (carbon) | 14 | 45.2% | 12.011 | 14 × 12.011 = 168.154 N (nitrogen) | 1 | 3.23% | 14.007 | 1 × 14.007 = 14.007 O (oxygen) | 1 | 3.23% | 15.999 | 1 × 15.999 = 15.999 F (fluorine) | 3 | 9.68% | 18.998403163 | 3 × 18.998403163 = 56.995209489 H (hydrogen) | 12 | 38.7% | 1.008 | 12 × 1.008 = 12.096 m = 168.154 u + 14.007 u + 15.999 u + 56.995209489 u + 12.096 u = 267.251209489 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction C (carbon) | 14 | 45.2% | 168.154/267.251209489 N (nitrogen) | 1 | 3.23% | 14.007/267.251209489 O (oxygen) | 1 | 3.23% | 15.999/267.251209489 F (fluorine) | 3 | 9.68% | 56.995209489/267.251209489 H (hydrogen) | 12 | 38.7% | 12.096/267.251209489 Check: 168.154/267.251209489 + 14.007/267.251209489 + 15.999/267.251209489 + 56.995209489/267.251209489 + 12.096/267.251209489 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent C (carbon) | 14 | 45.2% | 168.154/267.251209489 × 100% = 62.92% N (nitrogen) | 1 | 3.23% | 14.007/267.251209489 × 100% = 5.241% O (oxygen) | 1 | 3.23% | 15.999/267.251209489 × 100% = 5.987% F (fluorine) | 3 | 9.68% | 56.995209489/267.251209489 × 100% = 21.33% H (hydrogen) | 12 | 38.7% | 12.096/267.251209489 × 100% = 4.526%](../image_source/07137cab7f6126d603b30cb654608c32.png)
Find the elemental composition for 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde in terms of the atom and mass percents: atom percent = N_i/N_atoms × 100% mass percent = (N_im_i)/m × 100% Plan: • Write the chemical formula and gather atomic masses from the periodic table. • Determine values for N_i, m_i, N_atoms and m using these items. • Finally, compute the percents and check the results. Write the chemical formula: C_14H_12F_3NO Use the chemical formula to count the number of atoms, N_i, for each element and find the total number of atoms, N_atoms, per molecule: | number of atoms C (carbon) | 14 N (nitrogen) | 1 O (oxygen) | 1 F (fluorine) | 3 H (hydrogen) | 12 N_atoms = 14 + 1 + 1 + 3 + 12 = 31 Divide each N_i by N_atoms to calculate atom fractions. Then use the property that atom fractions must sum to one to check the work: | number of atoms | atom fraction C (carbon) | 14 | 14/31 N (nitrogen) | 1 | 1/31 O (oxygen) | 1 | 1/31 F (fluorine) | 3 | 3/31 H (hydrogen) | 12 | 12/31 Check: 14/31 + 1/31 + 1/31 + 3/31 + 12/31 = 1 Compute atom percents using the atom fractions: | number of atoms | atom percent C (carbon) | 14 | 14/31 × 100% = 45.2% N (nitrogen) | 1 | 1/31 × 100% = 3.23% O (oxygen) | 1 | 1/31 × 100% = 3.23% F (fluorine) | 3 | 3/31 × 100% = 9.68% H (hydrogen) | 12 | 12/31 × 100% = 38.7% Look up the atomic mass, m_i, in unified atomic mass units, u, for each element in the periodic table: | number of atoms | atom percent | atomic mass/u C (carbon) | 14 | 45.2% | 12.011 N (nitrogen) | 1 | 3.23% | 14.007 O (oxygen) | 1 | 3.23% | 15.999 F (fluorine) | 3 | 9.68% | 18.998403163 H (hydrogen) | 12 | 38.7% | 1.008 Multiply N_i by m_i to compute the mass for each element. Then sum those values to compute the molecular mass, m: | number of atoms | atom percent | atomic mass/u | mass/u C (carbon) | 14 | 45.2% | 12.011 | 14 × 12.011 = 168.154 N (nitrogen) | 1 | 3.23% | 14.007 | 1 × 14.007 = 14.007 O (oxygen) | 1 | 3.23% | 15.999 | 1 × 15.999 = 15.999 F (fluorine) | 3 | 9.68% | 18.998403163 | 3 × 18.998403163 = 56.995209489 H (hydrogen) | 12 | 38.7% | 1.008 | 12 × 1.008 = 12.096 m = 168.154 u + 14.007 u + 15.999 u + 56.995209489 u + 12.096 u = 267.251209489 u Divide the mass for each element by m to calculate mass fractions. Then use the property that mass fractions must sum to one to check the work: | number of atoms | atom percent | mass fraction C (carbon) | 14 | 45.2% | 168.154/267.251209489 N (nitrogen) | 1 | 3.23% | 14.007/267.251209489 O (oxygen) | 1 | 3.23% | 15.999/267.251209489 F (fluorine) | 3 | 9.68% | 56.995209489/267.251209489 H (hydrogen) | 12 | 38.7% | 12.096/267.251209489 Check: 168.154/267.251209489 + 14.007/267.251209489 + 15.999/267.251209489 + 56.995209489/267.251209489 + 12.096/267.251209489 = 1 Compute mass percents using the mass fractions: Answer: | | | number of atoms | atom percent | mass percent C (carbon) | 14 | 45.2% | 168.154/267.251209489 × 100% = 62.92% N (nitrogen) | 1 | 3.23% | 14.007/267.251209489 × 100% = 5.241% O (oxygen) | 1 | 3.23% | 15.999/267.251209489 × 100% = 5.987% F (fluorine) | 3 | 9.68% | 56.995209489/267.251209489 × 100% = 21.33% H (hydrogen) | 12 | 38.7% | 12.096/267.251209489 × 100% = 4.526%
Elemental oxidation states
![The first step in finding the oxidation states (or oxidation numbers) in 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: In 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde hydrogen is not bonded to a metal with lower electronegativity, so it will have an oxidation state of +1. Any element bonded to hydrogen gains the bonding electrons, decreasing their oxidation state by 1 for every bond: With hydrogen out of the way, look at the remaining bonds. There are 3 carbon-fluorine bonds, 3 carbon-nitrogen bonds, 1 carbon-oxygen bond, and 13 carbon-carbon bonds. For each of these bonds, assign the bonding electrons to the most electronegative element. First examine the carbon-fluorine bonds: element | electronegativity (Pauling scale) | C | 2.55 | F | 3.98 | | | Since fluorine is more electronegative than carbon, the electrons in these bonds will go to fluorine. Decrease the oxidation number for fluorine in every highlighted bond (by 1 for single bonds, 2 for double bonds, and 3 for triple bonds), and increase the oxidation number for carbon accordingly: Next look at the carbon-nitrogen bonds: element | electronegativity (Pauling scale) | C | 2.55 | N | 3.04 | | | Since nitrogen is more electronegative than carbon, the electrons in these bonds will go to nitrogen: Next look at the carbon-oxygen bond: element | electronegativity (Pauling scale) | C | 2.55 | O | 3.44 | | | Since oxygen is more electronegative than carbon, the electrons in this bond will go to oxygen: Next look at the carbon-carbon bonds: element | electronegativity (Pauling scale) | C | 2.55 | C | 2.55 | | | Since these elements are the same the bonding electrons are shared equally, and there is no change to the oxidation states: Now summarize the results: Answer: | | oxidation state | element | count -3 | C (carbon) | 2 | N (nitrogen) | 1 -2 | O (oxygen) | 1 -1 | C (carbon) | 5 | F (fluorine) | 3 0 | C (carbon) | 2 +1 | C (carbon) | 4 | H (hydrogen) | 12 +3 | C (carbon) | 1](../image_source/d1d6cda33397ed64b4ce3118fc4a9bab.png)
The first step in finding the oxidation states (or oxidation numbers) in 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde is to draw the structure diagram. Next set every oxidation number equal to the atom's formal charge: In 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde hydrogen is not bonded to a metal with lower electronegativity, so it will have an oxidation state of +1. Any element bonded to hydrogen gains the bonding electrons, decreasing their oxidation state by 1 for every bond: With hydrogen out of the way, look at the remaining bonds. There are 3 carbon-fluorine bonds, 3 carbon-nitrogen bonds, 1 carbon-oxygen bond, and 13 carbon-carbon bonds. For each of these bonds, assign the bonding electrons to the most electronegative element. First examine the carbon-fluorine bonds: element | electronegativity (Pauling scale) | C | 2.55 | F | 3.98 | | | Since fluorine is more electronegative than carbon, the electrons in these bonds will go to fluorine. Decrease the oxidation number for fluorine in every highlighted bond (by 1 for single bonds, 2 for double bonds, and 3 for triple bonds), and increase the oxidation number for carbon accordingly: Next look at the carbon-nitrogen bonds: element | electronegativity (Pauling scale) | C | 2.55 | N | 3.04 | | | Since nitrogen is more electronegative than carbon, the electrons in these bonds will go to nitrogen: Next look at the carbon-oxygen bond: element | electronegativity (Pauling scale) | C | 2.55 | O | 3.44 | | | Since oxygen is more electronegative than carbon, the electrons in this bond will go to oxygen: Next look at the carbon-carbon bonds: element | electronegativity (Pauling scale) | C | 2.55 | C | 2.55 | | | Since these elements are the same the bonding electrons are shared equally, and there is no change to the oxidation states: Now summarize the results: Answer: | | oxidation state | element | count -3 | C (carbon) | 2 | N (nitrogen) | 1 -2 | O (oxygen) | 1 -1 | C (carbon) | 5 | F (fluorine) | 3 0 | C (carbon) | 2 +1 | C (carbon) | 4 | H (hydrogen) | 12 +3 | C (carbon) | 1
Orbital hybridization
![First draw the structure diagram for 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Assign the provisional hybridization based on the table: Next identify any sp^3 atoms with lone pair electrons which can participate in a conjugated π-bond system. These atoms can lower their energy by placing a lone pair in a unhybridized p orbital to maximize overlap with the neighboring π-bonds. Note that halogens and elements from the third period and below do not engage in bond conjugation, except in the case of aromaticity: Adjust the provisional hybridizations to arrive at the result: Answer: | |](../image_source/be425e9a622ef9aafbcabe9c0de9d201.png)
First draw the structure diagram for 2, 5-dimethyl-1-[3-(trifluoromethyl)phenyl]pyrrole-3-carboxaldehyde, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Assign the provisional hybridization based on the table: Next identify any sp^3 atoms with lone pair electrons which can participate in a conjugated π-bond system. These atoms can lower their energy by placing a lone pair in a unhybridized p orbital to maximize overlap with the neighboring π-bonds. Note that halogens and elements from the third period and below do not engage in bond conjugation, except in the case of aromaticity: Adjust the provisional hybridizations to arrive at the result: Answer: | |
Topological indices
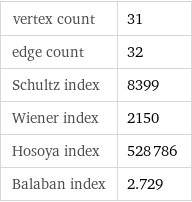
vertex count | 31 edge count | 32 Schultz index | 8399 Wiener index | 2150 Hosoya index | 528786 Balaban index | 2.729