Input interpretation
dibromoaurate(I) anion | cyanide anion | sulfate anion
Structure diagrams
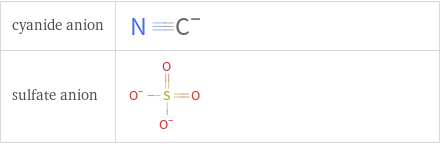
Structure diagrams
General properties
![| dibromoaurate(I) anion | cyanide anion | sulfate anion formula | ([AuBr_2])^- | (CN)^- | (SO_4)^(2-) net ionic charge | -1 | -1 | -2 alternate names | dibromogold(I) | dibromoaurate(I) | dibromoaurate(1-) | cyanide | cyanide(1-) | nitrile anion | tetraoxosulfate | tetraoxidosulfate | sulfate | sulfate(2-)](../image_source/8666fa440833931fe382fe0458dd2cd9.png)
| dibromoaurate(I) anion | cyanide anion | sulfate anion formula | ([AuBr_2])^- | (CN)^- | (SO_4)^(2-) net ionic charge | -1 | -1 | -2 alternate names | dibromogold(I) | dibromoaurate(I) | dibromoaurate(1-) | cyanide | cyanide(1-) | nitrile anion | tetraoxosulfate | tetraoxidosulfate | sulfate | sulfate(2-)
Ionic radii

| cyanide anion | sulfate anion thermochemical radius | 191 pm | 258 pm
Units

Other properties
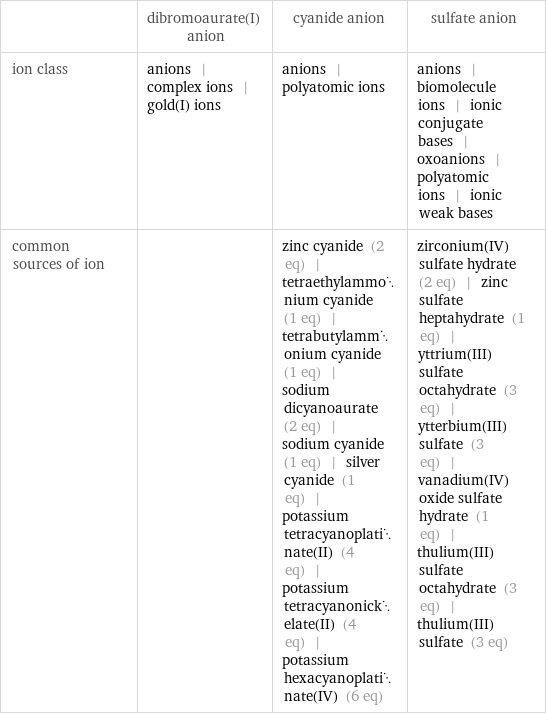
| dibromoaurate(I) anion | cyanide anion | sulfate anion ion class | anions | complex ions | gold(I) ions | anions | polyatomic ions | anions | biomolecule ions | ionic conjugate bases | oxoanions | polyatomic ions | ionic weak bases common sources of ion | | zinc cyanide (2 eq) | tetraethylammonium cyanide (1 eq) | tetrabutylammonium cyanide (1 eq) | sodium dicyanoaurate (2 eq) | sodium cyanide (1 eq) | silver cyanide (1 eq) | potassium tetracyanoplatinate(II) (4 eq) | potassium tetracyanonickelate(II) (4 eq) | potassium hexacyanoplatinate(IV) (6 eq) | zirconium(IV) sulfate hydrate (2 eq) | zinc sulfate heptahydrate (1 eq) | yttrium(III) sulfate octahydrate (3 eq) | ytterbium(III) sulfate (3 eq) | vanadium(IV) oxide sulfate hydrate (1 eq) | thulium(III) sulfate octahydrate (3 eq) | thulium(III) sulfate (3 eq)