Input interpretation
strontium chloride
Chemical names and formulas
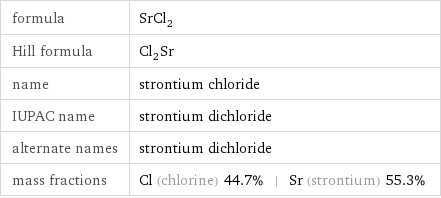
formula | SrCl_2 Hill formula | Cl_2Sr name | strontium chloride IUPAC name | strontium dichloride alternate names | strontium dichloride mass fractions | Cl (chlorine) 44.7% | Sr (strontium) 55.3%
Structure diagram
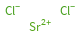
Structure diagram
Basic properties
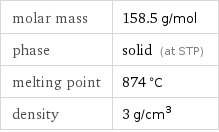
molar mass | 158.5 g/mol phase | solid (at STP) melting point | 874 °C density | 3 g/cm^3
Units

Solid properties (at STP)

density | 3 g/cm^3
Units

Thermodynamic properties
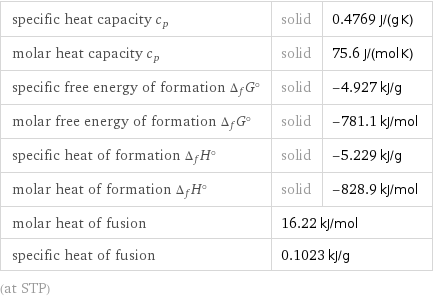
specific heat capacity c_p | solid | 0.4769 J/(g K) molar heat capacity c_p | solid | 75.6 J/(mol K) specific free energy of formation Δ_fG° | solid | -4.927 kJ/g molar free energy of formation Δ_fG° | solid | -781.1 kJ/mol specific heat of formation Δ_fH° | solid | -5.229 kJ/g molar heat of formation Δ_fH° | solid | -828.9 kJ/mol molar heat of fusion | 16.22 kJ/mol | specific heat of fusion | 0.1023 kJ/g | (at STP)
Chemical identifiers
![CAS number | 10476-85-4 PubChem CID number | 61520 PubChem SID number | 24867529 SMILES identifier | [Cl-].[Cl-].[Sr+2] InChI identifier | InChI=1/2ClH.Sr/h2*1H;/q;;+2/p-2/f2Cl.Sr/h2*1h;/q2*-1;m RTECS number | WK8400000 MDL number | MFCD00011249](../image_source/1775930c3efd82eb0ebcfb469ed15c1d.png)
CAS number | 10476-85-4 PubChem CID number | 61520 PubChem SID number | 24867529 SMILES identifier | [Cl-].[Cl-].[Sr+2] InChI identifier | InChI=1/2ClH.Sr/h2*1H;/q;;+2/p-2/f2Cl.Sr/h2*1h;/q2*-1;m RTECS number | WK8400000 MDL number | MFCD00011249
Ion equivalents

Sr^(2+) (strontium cation) | 1 Cl^- (chloride anion) | 2