Input interpretation
mercuric chloride | ammonium disulfatonickelate(II)
Chemical names and formulas
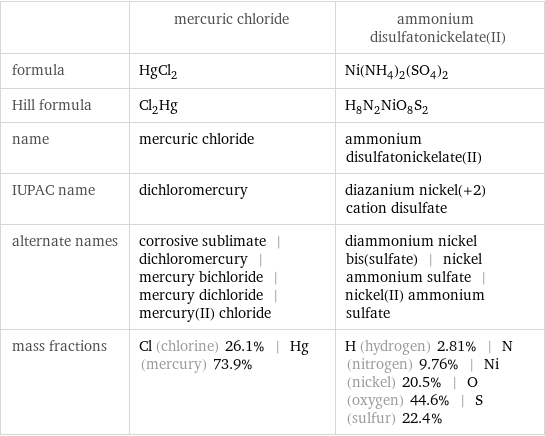
| mercuric chloride | ammonium disulfatonickelate(II) formula | HgCl_2 | Ni(NH_4)_2(SO_4)_2 Hill formula | Cl_2Hg | H_8N_2NiO_8S_2 name | mercuric chloride | ammonium disulfatonickelate(II) IUPAC name | dichloromercury | diazanium nickel(+2) cation disulfate alternate names | corrosive sublimate | dichloromercury | mercury bichloride | mercury dichloride | mercury(II) chloride | diammonium nickel bis(sulfate) | nickel ammonium sulfate | nickel(II) ammonium sulfate mass fractions | Cl (chlorine) 26.1% | Hg (mercury) 73.9% | H (hydrogen) 2.81% | N (nitrogen) 9.76% | Ni (nickel) 20.5% | O (oxygen) 44.6% | S (sulfur) 22.4%
Structure diagrams
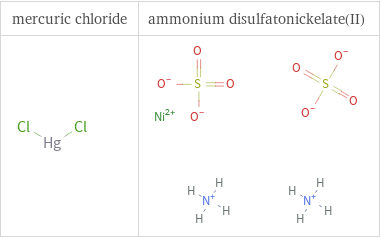
Structure diagrams
Basic properties
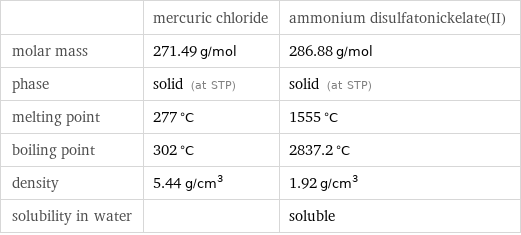
| mercuric chloride | ammonium disulfatonickelate(II) molar mass | 271.49 g/mol | 286.88 g/mol phase | solid (at STP) | solid (at STP) melting point | 277 °C | 1555 °C boiling point | 302 °C | 2837.2 °C density | 5.44 g/cm^3 | 1.92 g/cm^3 solubility in water | | soluble
Units

Thermodynamic properties

| mercuric chloride molar heat of fusion | 19.41 kJ/mol (kilojoules per mole) critical temperature | 973 K (kelvins)
Solid properties (at STP)

| mercuric chloride | ammonium disulfatonickelate(II) density | 5.44 g/cm^3 | 1.92 g/cm^3 vapor pressure | 1.3 mmHg |
Units

Chemical identifiers
![| mercuric chloride | ammonium disulfatonickelate(II) CAS number | 7487-94-7 | 15699-18-0 PubChem CID number | 24085 | 27455 PubChem SID number | 24852198 | SMILES identifier | Cl[Hg]Cl | [NH4+].[NH4+].[O-]S(=O)(=O)[O-].[O-]S(=O)(=O)[O-].[Ni+2] InChI identifier | InChI=1/2ClH.Hg/h2*1H;/q;;+2/p-2/f2Cl.Hg/h2*1h;/q2*-1;m | InChI=1/2H3N.Ni.2H2O4S/c;;;2*1-5(2, 3)4/h2*1H3;;2*(H2, 1, 2, 3, 4)/q;;+2;;/p-2/f2H4N.Ni.2O4S/h2*1H;;;/q2*+1;m;2*-2 EU number | | 239-793-5 Gmelin number | | 137483 RTECS number | OV9100000 | WS6050000 MDL number | MFCD00011041 |](../image_source/72fe51f40dff032760eaf9f79a92da37.png)
| mercuric chloride | ammonium disulfatonickelate(II) CAS number | 7487-94-7 | 15699-18-0 PubChem CID number | 24085 | 27455 PubChem SID number | 24852198 | SMILES identifier | Cl[Hg]Cl | [NH4+].[NH4+].[O-]S(=O)(=O)[O-].[O-]S(=O)(=O)[O-].[Ni+2] InChI identifier | InChI=1/2ClH.Hg/h2*1H;/q;;+2/p-2/f2Cl.Hg/h2*1h;/q2*-1;m | InChI=1/2H3N.Ni.2H2O4S/c;;;2*1-5(2, 3)4/h2*1H3;;2*(H2, 1, 2, 3, 4)/q;;+2;;/p-2/f2H4N.Ni.2O4S/h2*1H;;;/q2*+1;m;2*-2 EU number | | 239-793-5 Gmelin number | | 137483 RTECS number | OV9100000 | WS6050000 MDL number | MFCD00011041 |
NFPA label

NFPA label
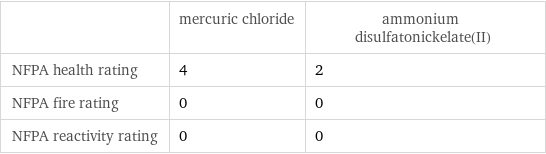
| mercuric chloride | ammonium disulfatonickelate(II) NFPA health rating | 4 | 2 NFPA fire rating | 0 | 0 NFPA reactivity rating | 0 | 0