Input interpretation
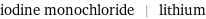
iodine monochloride | lithium
Chemical names and formulas
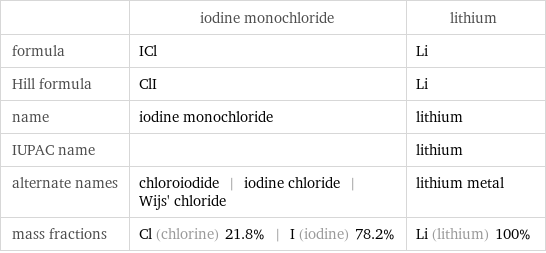
| iodine monochloride | lithium formula | ICl | Li Hill formula | ClI | Li name | iodine monochloride | lithium IUPAC name | | lithium alternate names | chloroiodide | iodine chloride | Wijs' chloride | lithium metal mass fractions | Cl (chlorine) 21.8% | I (iodine) 78.2% | Li (lithium) 100%
Structure diagrams
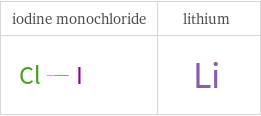
Structure diagrams
Basic properties
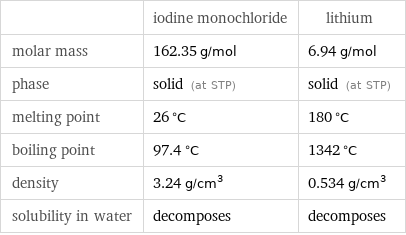
| iodine monochloride | lithium molar mass | 162.35 g/mol | 6.94 g/mol phase | solid (at STP) | solid (at STP) melting point | 26 °C | 180 °C boiling point | 97.4 °C | 1342 °C density | 3.24 g/cm^3 | 0.534 g/cm^3 solubility in water | decomposes | decomposes
Units

Drug interactions
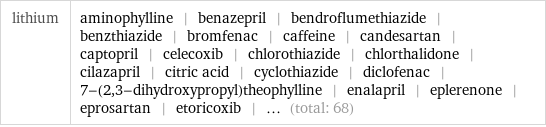
lithium | aminophylline | benazepril | bendroflumethiazide | benzthiazide | bromfenac | caffeine | candesartan | captopril | celecoxib | chlorothiazide | chlorthalidone | cilazapril | citric acid | cyclothiazide | diclofenac | 7-(2, 3-dihydroxypropyl)theophylline | enalapril | eplerenone | eprosartan | etoricoxib | ... (total: 68)
Thermodynamic properties
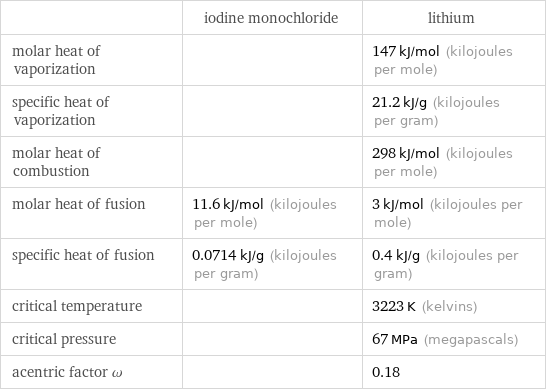
| iodine monochloride | lithium molar heat of vaporization | | 147 kJ/mol (kilojoules per mole) specific heat of vaporization | | 21.2 kJ/g (kilojoules per gram) molar heat of combustion | | 298 kJ/mol (kilojoules per mole) molar heat of fusion | 11.6 kJ/mol (kilojoules per mole) | 3 kJ/mol (kilojoules per mole) specific heat of fusion | 0.0714 kJ/g (kilojoules per gram) | 0.4 kJ/g (kilojoules per gram) critical temperature | | 3223 K (kelvins) critical pressure | | 67 MPa (megapascals) acentric factor ω | | 0.18
Solid properties (at STP)

| iodine monochloride | lithium density | 3.24 g/cm^3 | 0.534 g/cm^3 vapor pressure | 30 mmHg | 1 mmHg
Units

Chemical identifiers
![| iodine monochloride | lithium CAS number | 7790-99-0 | 7439-93-2 PubChem CID number | 24640 | 3028194 PubChem SID number | 24871766 | 24853199 SMILES identifier | ClI | [Li]](../image_source/ba2f24db749c457e0354b3c759ad1879.png)
| iodine monochloride | lithium CAS number | 7790-99-0 | 7439-93-2 PubChem CID number | 24640 | 3028194 PubChem SID number | 24871766 | 24853199 SMILES identifier | ClI | [Li]
NFPA label

NFPA label