Input interpretation
magnesium hydride
Chemical names and formulas

formula | MgH_2 Hill formula | H_2Mg name | magnesium hydride alternate names | magnesium dihydride | magnesium hydride ni-doped | tego magnan mass fractions | H (hydrogen) 7.66% | Mg (magnesium) 92.3%
Structure diagram
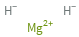
Structure diagram
Basic properties
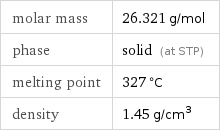
molar mass | 26.321 g/mol phase | solid (at STP) melting point | 327 °C density | 1.45 g/cm^3
Units

Solid properties (at STP)

density | 1.45 g/cm^3
Units

Thermodynamic properties
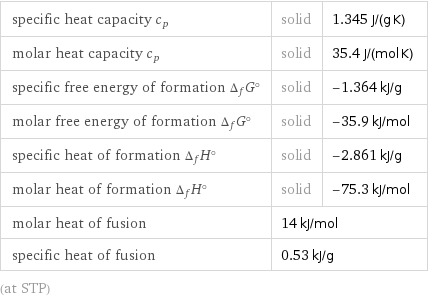
specific heat capacity c_p | solid | 1.345 J/(g K) molar heat capacity c_p | solid | 35.4 J/(mol K) specific free energy of formation Δ_fG° | solid | -1.364 kJ/g molar free energy of formation Δ_fG° | solid | -35.9 kJ/mol specific heat of formation Δ_fH° | solid | -2.861 kJ/g molar heat of formation Δ_fH° | solid | -75.3 kJ/mol molar heat of fusion | 14 kJ/mol | specific heat of fusion | 0.53 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7693-27-8 PubChem CID number | 107663 SMILES identifier | [H-].[H-].[Mg+2] InChI identifier | InChI=1/Mg.2H/q+2;2*-1 MDL number | MFCD00011101](../image_source/b85c73b0c4802b13f83c6afed5c3887f.png)
CAS number | 7693-27-8 PubChem CID number | 107663 SMILES identifier | [H-].[H-].[Mg+2] InChI identifier | InChI=1/Mg.2H/q+2;2*-1 MDL number | MFCD00011101
NFPA label

NFPA label

NFPA hazards | water reactive
Ion equivalents
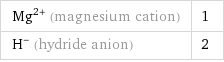
Mg^(2+) (magnesium cation) | 1 H^- (hydride anion) | 2