Input interpretation
complex ions
Members

tetrahydroxoberyllate anion | hexaaquamanganese(II) cation | hexaaquacobalt(II) cation | hexaaquanickel(II) cation | ferricyanide anion | ferrocyanide anion | dicyanoaurate(I) anion | hexaamminemanganese(II) cation | hexaamminecobalt(II) cation | pentaamminechlorocobalt(II) cation | hexaamminenickel(II) cation | diamminesilver(I) cation | tetrachloroborate anion | tetrachloroaluminate anion | tetrachlorocuprate(II) anion | hexafluoroplatinate(IV) anion | hexachloroplatinate(IV) anion | hexaiodoplatinate(IV) anion | dichloroaurate(I) anion | dibromoaurate(I) anion (total: 20)
Ionic radii
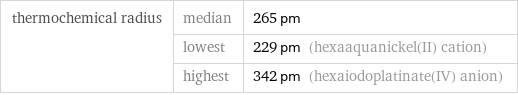
thermochemical radius | median | 265 pm | lowest | 229 pm (hexaaquanickel(II) cation) | highest | 342 pm (hexaiodoplatinate(IV) anion)
Units
