Input interpretation

halogens | atomic volume
Summary
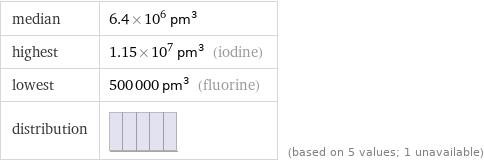
median | 6.4×10^6 pm^3 highest | 1.15×10^7 pm^3 (iodine) lowest | 500000 pm^3 (fluorine) distribution | | (based on 5 values; 1 unavailable)
Entity with missing value
tennessine
Ranked values
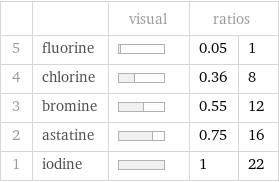
| | visual | ratios | 5 | fluorine | | 0.05 | 1 4 | chlorine | | 0.36 | 8 3 | bromine | | 0.55 | 12 2 | astatine | | 0.75 | 16 1 | iodine | | 1 | 22
Plot
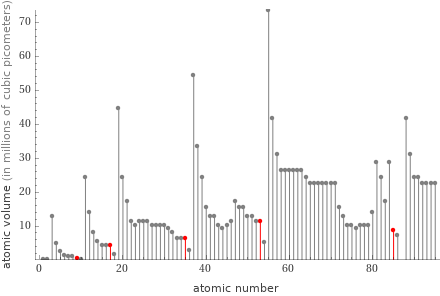
Plot
Periodic table location
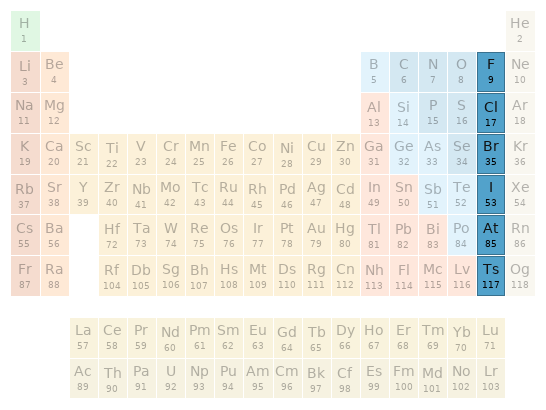
Periodic table location
Atomic radii properties
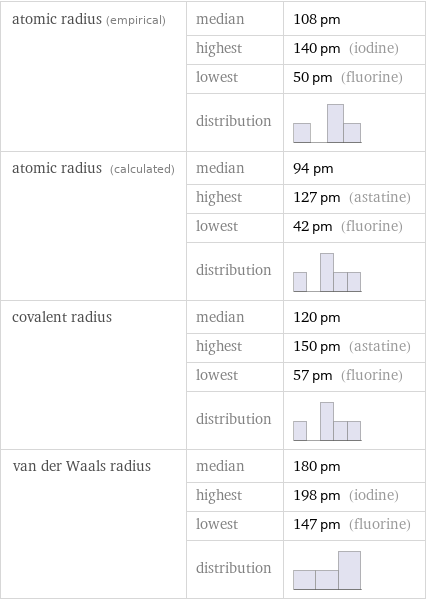
atomic radius (empirical) | median | 108 pm | highest | 140 pm (iodine) | lowest | 50 pm (fluorine) | distribution | atomic radius (calculated) | median | 94 pm | highest | 127 pm (astatine) | lowest | 42 pm (fluorine) | distribution | covalent radius | median | 120 pm | highest | 150 pm (astatine) | lowest | 57 pm (fluorine) | distribution | van der Waals radius | median | 180 pm | highest | 198 pm (iodine) | lowest | 147 pm (fluorine) | distribution |
Units

Atomic volume rankings
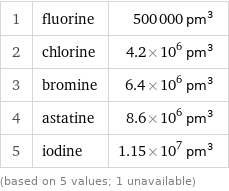
1 | fluorine | 500000 pm^3 2 | chlorine | 4.2×10^6 pm^3 3 | bromine | 6.4×10^6 pm^3 4 | astatine | 8.6×10^6 pm^3 5 | iodine | 1.15×10^7 pm^3 (based on 5 values; 1 unavailable)
Unit conversions for median atomic volume 6.4×10^6 pm^3
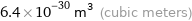
6.4×10^-30 m^3 (cubic meters)
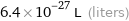
6.4×10^-27 L (liters)
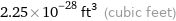
2.25×10^-28 ft^3 (cubic feet)
Comparisons for median atomic volume 6.4×10^6 pm^3

≈ 51 × volume of a helium atom ( 4 Pi/3 atomic radii of helium (the smallest atom)^3 )

≈ 97 × volume of a hydrogen atom ( 4 Pi/3 atomic radii of hydrogen^3 )
Corresponding quantities

Radius r of a sphere from V = 4πr^3/3: | 115 pm (picometers) | 4.5×10^-9 inches

Edge length a of a cube from V = a^3: | 185 pm (picometers) | 7.3×10^-9 inches