Input interpretation

alkaline earth metals | phase at STP
Summary
solid
Table
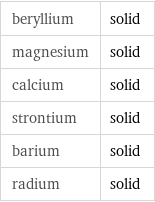
beryllium | solid magnesium | solid calcium | solid strontium | solid barium | solid radium | solid
Thermodynamic properties
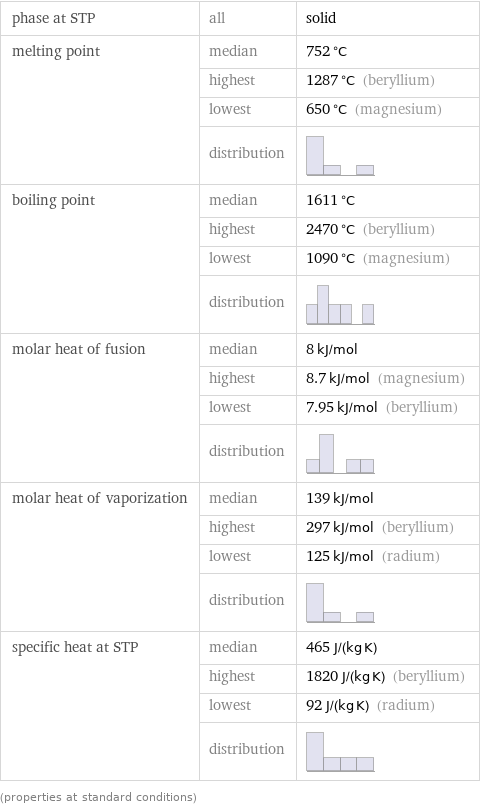
phase at STP | all | solid melting point | median | 752 °C | highest | 1287 °C (beryllium) | lowest | 650 °C (magnesium) | distribution | boiling point | median | 1611 °C | highest | 2470 °C (beryllium) | lowest | 1090 °C (magnesium) | distribution | molar heat of fusion | median | 8 kJ/mol | highest | 8.7 kJ/mol (magnesium) | lowest | 7.95 kJ/mol (beryllium) | distribution | molar heat of vaporization | median | 139 kJ/mol | highest | 297 kJ/mol (beryllium) | lowest | 125 kJ/mol (radium) | distribution | specific heat at STP | median | 465 J/(kg K) | highest | 1820 J/(kg K) (beryllium) | lowest | 92 J/(kg K) (radium) | distribution | (properties at standard conditions)