Input interpretation

carbon allotropes | molecular mass
Summary
![median | 12.011 u highest | 840.77 u ([5, 6]-fullerene-C70) lowest | 12.011 u (3 chemicals) distribution |](../image_source/401e4dbb9bb3f537238bde9c6cda5580.png)
median | 12.011 u highest | 840.77 u ([5, 6]-fullerene-C70) lowest | 12.011 u (3 chemicals) distribution |
Units

Ranked values
![| | visual | ratios | 5 | activated charcoal | | 0.014286 | 1 4 | diamond | | 0.014286 | 1 3 | graphite | | 0.014286 | 1 2 | buckminsterfullerene | | 0.8571 | 60 1 | [5, 6]-fullerene-C70 | | 1 | 70](../image_source/089aea58832774937ed81489d03bc4a6.png)
| | visual | ratios | 5 | activated charcoal | | 0.014286 | 1 4 | diamond | | 0.014286 | 1 3 | graphite | | 0.014286 | 1 2 | buckminsterfullerene | | 0.8571 | 60 1 | [5, 6]-fullerene-C70 | | 1 | 70
Molecular mass rankings
![1 | graphite | 12.011 u 2 | diamond | 12.011 u 3 | activated charcoal | 12.011 u 4 | buckminsterfullerene | 720.66 u 5 | [5, 6]-fullerene-C70 | 840.77 u](../image_source/2c21901e31911e17ae8ae38902513ba6.png)
1 | graphite | 12.011 u 2 | diamond | 12.011 u 3 | activated charcoal | 12.011 u 4 | buckminsterfullerene | 720.66 u 5 | [5, 6]-fullerene-C70 | 840.77 u
Unit conversions for median molecular mass 12.011 u
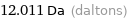
12.011 Da (daltons)
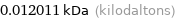
0.012011 kDa (kilodaltons)
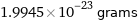
1.9945×10^-23 grams
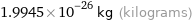
1.9945×10^-26 kg (kilograms)

12.012 chemical atomic mass units (unit officially deprecated)
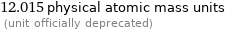
12.015 physical atomic mass units (unit officially deprecated)
Comparisons for median molecular mass 12.011 u
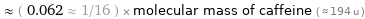
≈ ( 0.062 ≈ 1/16 ) × molecular mass of caffeine ( ≈ 194 u )
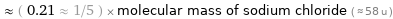
≈ ( 0.21 ≈ 1/5 ) × molecular mass of sodium chloride ( ≈ 58 u )
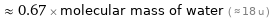
≈ 0.67 × molecular mass of water ( ≈ 18 u )
Corresponding quantities
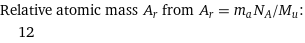
Relative atomic mass A_r from A_r = m_aN_A/M_u: | 12
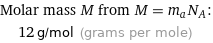
Molar mass M from M = m_aN_A: | 12 g/mol (grams per mole)
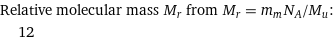
Relative molecular mass M_r from M_r = m_mN_A/M_u: | 12
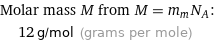
Molar mass M from M = m_mN_A: | 12 g/mol (grams per mole)