Input interpretation

2-iodophenol | orbital hybridization
Result
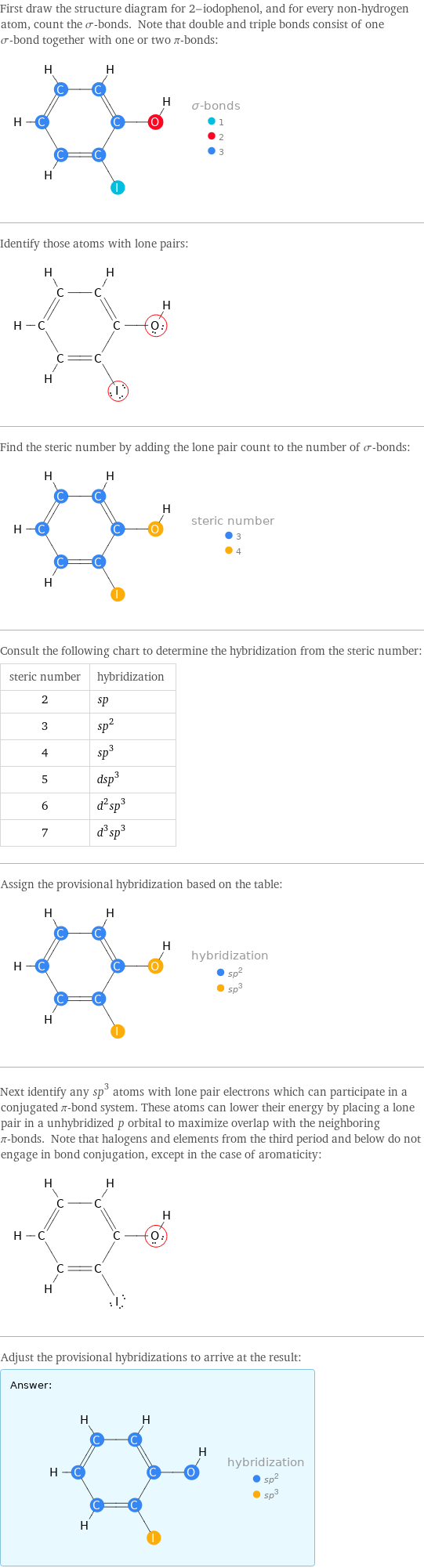
First draw the structure diagram for 2-iodophenol, and for every non-hydrogen atom, count the σ-bonds. Note that double and triple bonds consist of one σ-bond together with one or two π-bonds: Identify those atoms with lone pairs: Find the steric number by adding the lone pair count to the number of σ-bonds: Consult the following chart to determine the hybridization from the steric number: steric number | hybridization 2 | sp 3 | sp^2 4 | sp^3 5 | dsp^3 6 | d^2sp^3 7 | d^3sp^3 Assign the provisional hybridization based on the table: Next identify any sp^3 atoms with lone pair electrons which can participate in a conjugated π-bond system. These atoms can lower their energy by placing a lone pair in a unhybridized p orbital to maximize overlap with the neighboring π-bonds. Note that halogens and elements from the third period and below do not engage in bond conjugation, except in the case of aromaticity: Adjust the provisional hybridizations to arrive at the result: Answer: | |
Chemical names and formulas
formula | IC_6H_4OH Hill formula | C_6H_5IO name | 2-iodophenol alternate names | 2-jodfenol | o-iodophenol | phenol, 2-iodo- | phenol, o-iodo- mass fractions | C (carbon) 32.8% | H (hydrogen) 2.29% | I (iodine) 57.7% | O (oxygen) 7.27%